Cadmium Sulfide Nanoparticles
Cadmium sulfide nanoparticles and their quantum dot version are of a significant interest due to their semiconducting and luminescent properties, especially their controllable emission bands, continuous excitation and the ease of functionalization. Cadmium sulfide (CDS) is particularly used in devices with optoelectronic property and is known to be a direct band gap material at room temperature. Cadmium sulfide has attracted a lot of attention due to an extensive range of applications and unique properties. In fact, cadmium sulfide among the most widely studied semiconducting material with unique chemical and optical characteristics because of its quantum size effect. Particularly, cadmium sulfide has been shown to be a highly qualified photosensitive material and its perfect compatibility with organic semiconductors makes it a potential agent as an electron acceptor with an application in solar cells1.
Generally, cadmium sulfide is a yellow inorganic compound that occurs naturally as two distinct crystal lattices in the form of rare minerals and du to structural similarities, it is found in zinc ores as impurities as cadmium’s main economic source. With regard to the technical fact in nanotechnology, matter and mostly solids adopts specific, intensified, improved and novel properties when they fall into nano scale. In the case of semiconductor nanomaterials, the fundamental point to take into consideration is the development of efficient and reliable synthetic methods in order to obtain particles with appropriate size and morphology.
Physical and Chemical Properties of Cadmium Sulfide Nanoparticles
Similar to zinc sulfide, cadmium sulfide occurs in two crystalline forms with the hexagonal wurtzite as the most stable structure and the cubic zinc blende. Cadmium and sulfur atoms in these two crystalline forms adopt four coordination states. Cadmium sulfide is considered as a direct band gap semiconductor with its color being originated from visibility to electromagnetic radiation. Its conductivity increases with its irradiated and when it’s coupled with semiconductors of p-type, it can form the core component of solar or photovoltaic cells. It should be mentioned that CDS/Cu2S is among the first efficient solar cells introduced back in 1954. Cadmium sulfide adopts luminescent quality when it’s doped with Al3+ or Cu+ under electron beam excitation with applications in phosphorescence analyses. When cadmium sulfide is formed like a thin film, it adopts piezoelectric behavior to be used as transducers and can operate at Giga Hertz (GHz) region.
Synthesis and Preparation of Cadmium Sulfide Nanoparticles
Cadmium sulfide nanoparticle have been prepared based on methods such as Microwave-assisted solution precipitation synthesis, Chemical Vapor Deposition, solution precipitation, DC Electrochemical Deposition, gas phase reaction with hydrogen sulfide (H2S) or sulfur vapor, solvothermal and hydrothermal, and thermolysis process so far. Microwave assisted heating method has been used as an advantageous method with short reaction time and high energy efficiency as well as the possibility of inducing particles with smaller size, higher purity and narrower size distribution due to homogenous heating by microwave irradiation 1. On the contrary for solar cells, it is essential to investigate the physical and chemical properties as well as photovoltaic performance that are affected by preparation conditions of CDS synthesis. For an application in solar cells, cadmium sulfide nanoparticles are synthesized taking the advantage of microwave radiation and the solution precipitation method using thiourea (TU) and thioacetamide (TA) as the sources of sulfur. A solution containing cadmium chloride (CdCl2), sodium citrate (HOC(COONa)(CH2COONa)2) and KOH are prepared and the pH is adjusted to be around 7. The solution is heated up by microwave radiation up to 150°C and maximum 30 minutes until it changes to yellow. The resulting CDS should be washed and dried at room temperature 1.
Recently, a novel biological using capping biomolecules method has also been suggested to synthesize cadmium sulfide nanoparticles. Some studies have reported microbial synthesis of CDS nanoparticles using the E-Coli based on the concept that nanoscale crystals synthesis relies on cell’s growth phase. In a different biosynthetic method, bacterial protein has been employed to serve in the synthesis process and particle formation. Nanosized cadmium sulfide crystals synthesis has been prepared through genetic engineering using genes that are responsible to bind CDS to peptides. In general, biomolecules and microorganisms sustain a significant number of nucleation centers to control particle size, crystallinity and morphology of nanomaterials and make it possible to achieve highly stable and dispersed nanoparticles 2. Particularly in a biological method, the biosynthesis of CDS nanoparticles involves the use of microbial cells obtained from a logarithmic phase being in the mature and high dividing stage of their bacterial life cycle. The bacterial cells are centrifuged and washed in phosphate-buffered saline (PBS, pH 7.4) to ensure the removal of the bacterial culture media. CdCl2solution is added to buffer containing the bacteria and incubated for 12 hours at 37°C to obtain the desired cadmium sulfide nanoparticles 2.
In a methods called thermal decomposition, cadmium sulfide nanoparticles are synthesized by a novel Cd-octonate complex. In this order, CDS nanoparticles are synthesized under argon atmosphere, an amount of sulfur is added to Cd-octonate complex and heated at 170 °C 2 hours with a color change from green to black. The resulting solution is left to cool down to room temperature and the nanoparticles are separated and washed in ethanol and dried 3.
Applications of Cadmium Sulfide Nanoparticles
Cadmium sulfide nanoparticles are known for their extensive applications dual semiconducting and luminescence properties and show a great promise in medical imaging and treatment of disease. CDS nanoparticles have potential applications in laser light-emitting diodes, thin film transistors, electronic and non-linear optical optoelectronic devices, single-electron transistors, photo catalysis photo-electrochemical cells, solar cells, in electroluminescence analysis thin films and solid state lasers. In thin films, cadmium sulfide is combined with interactive layers for applications in some specific solar cells. They are also among the first semiconductor materials used widely in thin film transistors. Cadmium sulfide thin films exhibit piezoelectric behavior and are employed as transducers that can operate at Giga Hertz region of electromagnetic radiation. Nanoscale cadmium sulfide has shown to have a net cooling which originates from the annihilation of photons during anti-stock luminescence at wavelengths as small as 510 nm. Therefore, a maximum decline in temperature is recorded when nanoscale cadmium sulfide particles are pumped with a laser that functions at wavelengths like 514 and 532 nm.
Conclusion
The dual property and extensive applications have made cadmium sulfide nanoparticles as qualified nanomaterials to attract a lot of attention in industries, medicine and science. In addition to common physical and chemical methods, the synthesis methods fit in the standards of green chemistry due to the use of biomolecules like bacteria with applications in medicine.
References
1. Martínez-Alonso, C., Rodríguez-Castañeda, C. A., Moreno-Romero, P., Coria-Monroy, S. & Hu, H. Cadmium sulfide nanoparticles synthesized by microwave heating for hybrid solar cell applications. Int. J. Photoenergy 2014, 10–12 (2014).
2. Sankhla, A. et al. Biosynthesis and characterization of cadmium sulfide nanoparticles – An emphasis of zeta potential behavior due to capping. Mater. Chem. Phys. 170, 44–51 (2016).
3. Seyghalkar, H., Sabet, M. & Salavati-Niasari, M. Synthesis and Characterization of Cadmium Sulfide Nanoparticles via a Simple Thermal Decompose Method. High Temp. Mater. Process. 35, 1013–1016 (2016).

Hydroxyapatite Powder
Hydroxyapatite powder also called HA powder is a mineral present in either solid or crystalline form which is then converted into the powdered form. It is based on the presence of calcium, hydroxyl ions and phosphorus in different ratios. It is synthesized chemically in various forms having a balanced chemical equation with appropriate ratios. It has two biological functions too, mantis shrimp and humans/mammals/primates. It has several uses as well in the field of cosmetics, supplements, medical and archeology. With the world pacing at such a great rate, HA powder has proved to be beneficial in several ways. Though there are still some manufacturers who are claiming this mineral to be a misfit but on the other hand, it has multiple supporters too so that is why it is rightly believed that benefits and misfits go hand in hand and it is true in plenty of ways too because we have to be careful as to what product or use of HA powder will prove to be beneficial or harmful for us.
Introduction
Hydroxyapatite which is also known as hydroxylapatite (HA) is a form of a mineral calcium apatite which occurs naturally, the formula of which is Ca5(PO4)3(OH). It is therefore known that the mineral hydroxyapatite is a crystalline formed cell and has two separate structures. Hydroxyapatite is one of the members of the apatite group, which is a complex one. The hydroxyl ions can be alternated by carbonate, chloride or fluoride. Upon heating, it is crystallized and is present in the crystalline form which can then be converted into the powdered form by crushing the crystals of it that is why the pure form of it is in white colour. Although the ones which occur naturally might be present in other colours too, that is brown, yellow or green. A bone mineral which is present in a normal human bone is an altered form of the hydroxyapatite because fifty per cent of it by volume and seventy percent of it by weight is actually the bone mineral comprised of hydroxyapatite. The minute structures of hydroxyapatite are also found in the pineal gland, brain sand and a few other structures of the human body. These are the tiny calcifications of the human body.
Hydroxyapatite powder can be chemically synthesized in multiple different ways. Calcium, phosphorus and hydroxyl ions are the main components of a hydroxyapatite powder. They can be altered in various ways to bring out various shapes and forms of the powder, but the main formula and equation that formulates the process remain the same which are mentioned below respectfully.
It can be formed in solid-state too which later on is converted into the crystalline form and from that is converted into the powdered form to perform various functions for different purposes.
Properties of Hydroxyapatite powder
The most prominent property of hydroxyapatite powder is the level of its stability at the time of comparison with the other calcium phosphates. However, HA powder is believed to be that calcium phosphate compound which is the most stable under the influence of physical conditions such as the composition of body fluids, pH and temperature. Following are the exceptional chemical properties of HA powder:

Structure of Hydroxyapatite
Biocompatibility:
One of the excellent property of HA powder is biocompatibility. HA powder enhances the biocompatibility of all the products it is used in.
Bioactivity:
Another good property of HA powder is bioactivity. All the products or minerals which have HA powder extraction in them speeds up the bioactivity of those products or minerals.
Osteoconductivity:
Osteoconductivity too is one of the greatest properties of HA powder. As HA powder can either be transparent or opaque, so osteoconductivity is exceptionally enhanced in it.
Non-toxicity and non-inflammatory nature:
HA powder possesses the non-toxic and non-inflammatory nature that is why it can be used for various purposes as it has no harmful, toxic or inflammatory effects on any of the things it is mixed with.
Following are the physical properties of the HA powder:
1)Like all the other ceramics, HA is also very brittle.
2)The usual colours of HA powder are green, grey, red, brown, violet or colourless, but the colour of it depends upon the type of apatite.
3)It can be transparent or opaque.
4)It gets degraded in the solution of 2.0 pH.
Uses of hydroxyapatite powder
Hydroxyapatite powder is used in various fields for various purposes which are mentioned below as it is one of the most preferred minerals in the industry because of its remarkable benefits.
Uses of hydroxyapatite powder in Cosmetics
The products such as Johnson’s Aloe and Vitamin E powder are the ones which have HA powder in them. It is to be notified that these make the products help in either moisturizing the skin or softening the skin of the babies. It is also present in the makeup kits as their initial purpose is to provide moisture and softness to the skin. A few rare cases of HA powder being used in the shampoos too came out as to give protection to the dry and dull hair. It proved beneficial in the hair products too. These products are very reliable and are being sold and purchased at a very great rate in the market as they are extremely beneficial, and HA products are one of the main contents that make these products beneficial.
Uses of hydroxyapatite powder in Medical:
In the field of medical, HA powder is drastically used for unique bodily implantations like hip replacements, dental implants and bone conduction implants. A very fine coating of HA is done on these implants for the protection because this powder contains calcium and phosphorus. Even though, science is making progress in enhancing the credibility of the structure of the bones via replacement because these are very much needed for the proper functioning of a normal human body and if these will be missed out from the implants then there are more chances of the implants being unsuccessful.
Use of hydroxyapatite powder in supplements:
Hydroxyapatite powder is also present in another form that is the microcrystalline form, and this form of hydroxyapatite is very much known as a supplement that has a very high rate of absorbing as compared to calcium and is retailed as a bone-building mineral in the powdered form. It is mainly the supplement for calcium and has been derived from the bovine bone. Along with that, there are certain, and countless companies proclaim this that the HA powder used in the supplements is corrupted by the usage of heavy metals as its effective causes have not proved to be well in the field.
Use of hydroxyapatite powder in archaeology:
Hydroxyapatite powder is also being used in the field of archeology for the reconstruction of the migrants, diets and climatic changes of the human and animal remains. Bone and teeth in their mineral forms play the role of reservoirs of the trace elements, which also include carbon, oxygen and strontium. The hydroxyapatite powder is constructively used for the indication of the diet as in which state it is present, the habits which a human or animal adopt according to the geographical regions or origins and abruptly for that reconsolidation of the previous temperatures and the shifts of the climatic changes.
Use of hydroxyapatite powder in chromatography:
Hydroxyapatite powder is also being used in the process of chromatography. It is one of the very complex processes and is often also known as mixed mode. It basically represents the attraction and reaction of positive and negative ions which are then converted to ions. For the purpose of purification, HA chromatography’s efficacy cannot be predicted on the basis of physical and chemical properties it holds. For the purpose of elution, the buffers having a high quantity of phosphate or the neutral salt are most preferably used.
Use of hydroxyapatite powder in defluoridation:
Hydroxyapatite powder works as an excellent adsorptive mineral to complete the defluoridation of normal drinking water. This is due to the reason that HA powder can form fluorapatite in a process following three steps only. The mineral HA powder helps in the removal of fluoride ions from the water, and it then replaces those fluoride ions with the hydroxyl ions which actually makes fluorapatite which is incredibly useful in the purification of the contaminated water.
Use of hydroxyapatite powder in Mammals/Humans/Primates:
In mammals, humans or primates the hydroxyapatite is present at two different places. One is bone, and the other is teeth.
In bone, the matrix of collagen is sixty-five to seventy percent of the mass which is basically hydroxyapatite. In teeth, the matrix of dentin and enamel are seventy to eighty percent of the mass which is basically hydroxyapatite
All the studies show that the harmful effects associated with the usage of HA powder in all the different fields are very less and are almost unlikely to be recorded. Along with that, the studies also show briefly that the usage of HA powder that is the calcium phosphate nanoparticles are considered very safe for humans. Besides being safe, these products bring ease and efficacy too in the lives of humans so the usage of this mineral should be continued and various other ways should be explored in which humans can make it more beneficial for them.
Conclusion
In conclusion, Hydroxyapatite powder is a very complex and beneficial mineral found from this planet Earth. All the processes and procedures that are carried out in order to bring it out in its most useful forms are complicated in their own sense and require a lot of practice. Once it is presented in its purified and useful forms, it can be served for various helpful purposes, and in almost all sorts of fields, it has its own benefits. Off course there are conceptions like any other thing about HA powder too that it causes some harmful effects as well which are acceptable, but if we look at the brighter side, then we will see how it is so much more beneficial for us. It protects our bones and teeth so impeccably that it is the greatest example of its benefits itself.
References
https://www.sciencedirect.com/science/article/pii/…
https://www.medicinenet.com/script/main/art.asp?ar…
https://www.sciencedirect.com/topics/materials-sci…
https://www.fluidinova.com/hydroxyapatite-properti…
Antimony Tin Oxide Nanoparticles
Antimony doped tin oxide (ATO) nanoparticles have been proven to exhibit special optical property and excellent electrical conductivity to be used as thin film electrodes. Studies have shown that antimony doped tin oxide possesses a high n-type electric performance and employed in the form of the traditionally produced aerogel thin films for the purpose of collecting electrons in solar cells. Introducing antimony to the tin oxide structure can significantly increase the electron conductivity making it an excellent conductive material. Antimony tin oxide is transparent in the entire region of visible electromagnetic light, whereas it reflects the infra-red light. Generally, majority of the research projects concerning ATO have concentrated on the thin films of antimony doped tin oxide no matter they are made of nanoparticles or from particular precursors directly.
Physical and Chemical Properties of Antimony Tin Oxide Nanoparticles
ATO nanoparticles have attracted significant attention due to their well-known infrared light insulation quality, electrically conducting oxide and optical transparency. Antimony as the n-type dopant of tin oxide (SnO2) modifies the band structure of the tin oxide. Extra electron donation to the conduction band upon a cation substitutional replacement is carried on via a dopant as an impurity. The band structure of antimony doped tin oxide shows analytical data of Sb-5s-like band in the tin oxide’s band gap by a characteristic free electron at the G point. Additionally, it is elicited that this band gap is basically a half filled metallic ion with extra thermal excitation into bands similar to those of tin capable of increasing the conductivity 1. Antimony, which is the doped agent in antimony tin oxide has two ionic states based on its two valence electrons as Sb3+ and Sb5+ with a feasible switch Sb3+↔Sb5+ regarded as the redox enzymes catalytic cycle analogue in which the metal ions serve as cofactors in order to promote the possible reversing redox reactions against intracellular oxidizing agents.
Generally, transparent conducting oxides (TCOs) like antimony tin oxide and its nanoscale crystals combine transparency quality in the visible range of electromagnetic wave with high electrical conductivity making them considerable materials for several optoelectrochemcial applications. TCOs like antimony tin oxide nanoparticles are produced predominantly and extensively as thin layers and coatings. However, there has been a fast-growing trend and interest on obtaining nanosized crystals as small as possible in order to take the advantages of nanoparticles novel and improved properties that add to the bulky mother materials. The colloidal dispersion of antimony tin oxide nanoparticles are of a great interest for wet chemical deposition purposes. As the chemical and physical properties of materials like ATO nanoparticles directly depends of the size and morphology, synthesis of this class of agents is of a significant step for humidity sensing applications.
Synthesis and Preparation of Antimony Tin Oxide Nanoparticle
Considering the predominant applications of antimony tin oxide nanoparticles, numerous methods and techniques have been introduced to obtain them. Basically, it has to be taken into consideration that the properties of ATO is influenced by the synthesis and preparation process. The goal is always to obtain crystals with controlled size and an appropriate monodispersity so that high performance material is achieved. On the contrary, it is still challenging to synthesize antimony tin oxide nanoparticles combining all the required standards and determining factors like adequate conductivity, desirable particle size crystallinity, narrow particle size distribution and favorable dispersibility in a particular solvent. The most common and known methods include simple thermal evaporation, microemulsions, sol-gel, hydrothermal methods, co precipitation, mechanochemical, laser ablation, screen printing, combustion route, microwave assisted synthesis, thermal decomposition, hot injection and DC arc plasma jet synthesis.
In a synthesis procedure based chemical precipitation method, a solution of hydrochloric acid (HCl) is prepared and a mixture of SnCl2.H2O and SbCl2 with the ratio of 1:4 is added to acid solution with a consecutive dropwise addition of ammonium hydroxide to them. In the next step, an amount of polyethylene glycol as a capping agent is added to the mixture while vigorous magnetic stirring for around 24 hours until all the ingredients engage in a complete reaction. The resulting crystals are dried at 80°C for 8 hours and calcined at 400°C 2.
In a different method, the synthesis of antimony tin oxide nanoparticles is done via dissolving granulated tin (Sn) in nitric acid (HNO3). Separately, Antimony trioxide (Sb2O3) is dissolved in melted citric acid and is added to the previous solution and stirred for 3 hours at room temperature. After that, antimony hydroxide is added to the solution in order to process the precipitation of Sb3+ and Sn4+ metal ions and the pH of the solution is adjusted at 7. The hydroxide precipitate is washed in double distilled water until the color of the samples turn yellow. The resulting precipitates are dried at 100°C for 4 hours and calcined at 600°C for 2 hours in a muffle furnace essential to ensure the particles conductive property 3. A number of studies has focused on the assembly of antimony tin oxide nanoparticles into aerogels as big as a few centimeters with increasing applications as catalysis, photo catalysis and ferroelectric agents with promising properties like high porosity, high surface area to low density magnetic and optical quality. The assembly procedure is carried out by microwave heating and the assistance of non-aqueous sol gel reaction of antimony acetate Sb(CH3COO)3 with tin chloride (SnCl2) in the solvent made by mixing benzyl alcohol and toluene. The resulting mixture is heated up to 150°C for 9 minutes to obtain a brown precipitate 4.
Applications of Antimony Tin Oxide Nanoparticle
Referring to the unique electrical and conductive properties, antimony tin oxide (ATO) nanoparticles have been used extensively as transparent electrodes and thin films, support material for electro catalysis, energy storage devices, gas sensors and photoelectrocatalysis. It has been employed as a supporting agent in composite materials, radioactive waste management and optoelectronics. The antimony doped tin oxide nanoparticles have found broad applications in solar cells and heat reflection coatings too. Since ATO nanoparticles are transparent in visible region, they can be used as heat mirrors and transparent electrodes as well.
Conclusion
Antimony tin oxide nanoparticles quality as a transparent agent with favorable electrical conductivity has made it interesting nanoscale crystals in thin film electrode fabrication. In fact, most of the research has focused on optimizing the synthetic methods with applications in thin films production. The electron collecting quality of ATO nanoparticles has been drawing attention to their use in developing efficient solar cells.
References
1. Krishnakumar, T. et al. Structural, optical and electrical characterization of antimony-substituted tin oxide nanoparticles. J. Phys. Chem. Solids 70, 993–999 (2009).
2. Yadav, B. C., Singh, R., Singh, S., Kumar, R. & Srivastava, R. Nanostructured antimony tin oxide synthesized via chemical precipitation method : its characterization and application in humidity sensing. 1–16.
3. Zhang, J. & Gao, L. Synthesis and characterization of antimony-doped tin oxide (ATO) nanoparticles. Inorg. Chem. Commun. 7, 91–93 (2004).
4. Rechberger, F., Ilari, G. & Niederberger, M. Assembly of antimony doped tin oxide nanocrystals into conducting macroscopic aerogel monoliths. Chem. Commun. 50, 13138–13141 (2014).
C60 Fullerenes
After quite a few years since their discovery, fullerenes are now a well-established subject of research in many areas of chemistry and materials science. Years of studies have made researchers aware of both potentials and limitations of this class of compounds. Fullerene science plays a noticeable role in fields, such as Nanotechnology, Supramolecular Chemistry and Materials Chemistry. One of the most promising territories of investigation includes the modification of the fullerene cage to improve and/ or enhance its qualities for various applicative purposes since fullerenes possess unusual magnetic, optical, photophysical, electrochemical, semiconducting and superconducting properties. A new research field has aroused based on the functionalization and study of new fullerene types in recent years, which intends to benefit from the properties of these carbon hollow clusters in different technological fields. The high number of functionalization probabilities combined with the rich supramolecular chemistry of fullerenes provides the production of multiple derivatives of fullerenes with a wide diversity of architectures and these are continuously discovered as novel materials.
C60 fullerene (also called as Buckminsterfullerene) is a type of fullerene with the formula C60. It has a cage-like fused-ring that reminds of a soccer ball, and it is made of twenty hexagons and twelve pentagons, with a carbon atom which has one π bond and two σ bonds at each corner of the shape to create a universal vertex.
![Stereographic projection of C60 fullerene[1]. (Note: To visualize the 3D image, the picture must be held about 15 cm from the eyes and the eyes must focus at infinity). Stereographic projection of C60 fullerene[1]. (Note: To visualize the 3D image, the picture must be held about 15 cm from the eyes and the eyes must focus at infinity).](https://nanografi.com/product_images/uploaded_images/3235ecc664ddae45530b60f0c80e91ba.png)
Figure 1: Stereographic projection of C60 fullerene[1]. (Note: To visualize the 3D image, the picture must be held about 15 cm from the eyes and the eyes must focus at infinity).
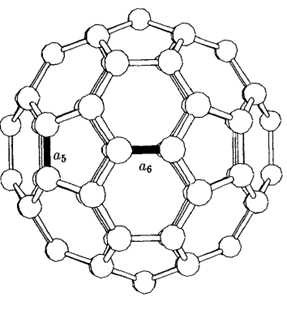
Figure 2: The C60 molecule showing single bonds (a5) and double bonds (a6) [2].
In the 1980s gas-phase studies, in which clusters and molecular species were produced by laser vaporization of a graphite target, mass spectroscopy was used as the main characterization tool for fullerenes. As might be expected, C60 fullerene was discovered in 1985 by Robert Curl, Harold Kroto, and Richard Smalley using laser evaporation of graphite. Hence, the closed cage nearly sphere shaped C60 fullerene have attracted a great interest in last decades due to its unique structure and properties.
Properties
Theoretically every material exhibits wave-particle ductility behavior, however the buckminsterfullerene (C60 fullerene) is the largest material that is observed to exhibit this behavior. The appearance of fullerenes differs according to the thickness of the material, whether or not they are in solution, and the nature of the solvent. Very thin films of C60 fullerene are mustard yellow, the color becoming brown, and finally black as the film thickness increases. Solutions of C60 fullerene have a beautiful magenta color in hydrocarbon solvents, but appear brown in some pi-donor solvents, due to probably some charge-transfer interactions.
Click Images to Read More about Fullerenes
Solubility
Accurate determination of solubilities is difficult, partly because saturated solutions are dark and opaque, hence it is not easy to see if all the solid fullerene has dissolved. Also, a curious feature is that the solubility passes through a maximum with increasing temperature. However, there is some evidence that the behavior of C60 fullerene in this respect depends upon whether or not the sample has been baked free of solvent prior to carrying out the temperature/ solubility measurements. This aspect is still under investigation, but it seems possible for example, that clustering (there is evidence that this exists in solution), may differ appreciably for baked- and unbaked samples. The close packing in baked samples could cause clusters to be solvated as such, the clusters then gradually breaking up as the temperature of the solution is raised. The solubilities of C60 fullerene have been determined in studies and the results have been discussed over the years. It has been concluded that it is evident that there is no solvent parameter which universally explains the solubility of C60 fullerene. There does seem to be an increase in solubility with increase in solvent molecular size, but any correlation with electron-donating abilities of the solvent is lacking.
Oxidizing Properties and Toxicity
C60 fullerene is a potent producer of singlet oxygen and therefore precautions should be taken to avoid contact with the skin. However, in this connection it should be noted that whilst a water-soluble fullerene carboxylic acid showed biological activity-toxicity and G-selective DNA cleaving ability (but only under the action of low-level visible light) and that fullerenes are potentially excellent photoinduced electron-transport moieties C60 fullerene has been found not to affect the proliferation of human keratinocytes or human fibroblasts indicating that its accumulation in human cells does not result in acute toxicity. Also, under visible or UV irradiation in the presence of molecular oxygen, alkenes and dienes undergo reaction with singlet oxygen if a trace of fullerene is present to give photo-oxygenation products. There is however a disagreement as to the relative effectiveness of the fullerene, some surveys find the former to be the more efficient catalyst, whilst others find it to be the latter. Because C60 fullerene has a low-lying LUMO, it is readily reduced and can acquire, reversibly, up to six electrons under electrochemical conditions. The potentials at which the electrons are added depends on the extent to which the solvent either donates electrons to the cage or accepts them from it. Therefore, C60 fullerene has good oxidizing properties and may oxidize readily the other materials in which it is contact.
Physical and Thermal Properties
Some useful data for C60 fullerene are the following:
Mean diameter, 6.83 4 outer diameter, 10.18 A; inner diameter, 3.48 A; average C-C distance 1.44 A FCC (face-centered cubic) lattice constant 14.17 A. Mass density, 1.72 g cm-1; molecular density, 1.44 x 1021 cm-3;; thermal conductivity (at 300 K), 0.4 W mK-1; phase transition temperatures, 90 K, 260 K. Binding energy per atom, 7.4 eV; electron affinity, 2.65 eV, 1st ionization potential, 7.58 eV; 2nd ionization potential, 11.5 eV; HOMO-LUMO band gap, 1.7 eV.
Production
Three methods are commonly used to make C60 fullerenes. These are:
(i) The Hufmann–Kratschmer procedure involving arc-discharge between
graphite rods in an atmosphere of helium.
(ii) Combustion of benzene in a deficiency of oxygen.
(iii) Condensation of polycyclic aromatic hydrocarbons through pyrolytic
dehydrogenation or dehydrohalogenation
The Hufmann–Kratschmer Method
Hufmann-Kratschmer method has been the most important method for fullerene production, and its introduction marked the real beginning of fullerene science. It is the preferred method because the only by-product is graphite, which if necessary, can be reformed into rods and recycled. The Hufmann–Kratschmer method involves arc-discharge between high-purity carbons rods of 6 mm diameter in an atmosphere of 100–200 torr helium. Argon may also be used but is less effective. The temperature required for fullerene formation is 2000 ℃, and obviously a small gap between the rods is necessary to prevent a fall in temperature. The need for this gap was shown at an early stage and was confirmed subsequently. The yield of fullerenes in the soot produced is approximately 5% and is higher when taken from the reactor at greater distances from the arc source, which implies that the initially formed fullerenes are subsequently degraded by UV irradiation. Numerous ingenious variations in reactor design were introduced at an early stage, including, for example, a carousel and one with an autoloading device. The latter method, designed by Bezmelnitsyn and Eletskii is especially notable, using carbon strips cut from a reactor moderator block. A stack of 24 of these (anode) are housed in a water-cooled chamber equipped with a hinged door fitted with an O-ring seal. The cathode consists of slowly rotating a carbon wheel which passes a scraper to remove accumulated slag. The strips are gravity fed and the lowest strip is slowly wheel-driven into the cathode. When consumed it drops away exposing the next strip, and the process continues during 24 hours to yield 100–200 g of fullerene-containing soot, accessed by opening the end door of the reactor.
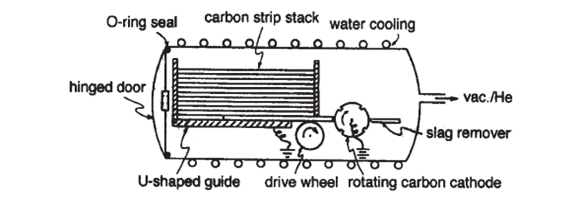
Figure 3: Schematic depiction of an autoloading version of an arc-discharge apparatus
used for C60 fullerene production[3].
The Combustion Process
Scientists have discovered that combustion of benzene in a deficiency of oxygen resulted in the formation of C60 fullerenes. This continuous method has been developed to the extent that a purpose-built factory has been erected in Japan, capable of producing 5000 ton of fullerenes per year, but currently running at about one-tenth of that capacity. Hence, one envisages that this investment must be driven by the expectation or knowledge that large-scale applications of fullerene lie ahead.
Condensation of Polycyclic Aromatic Hydrocarbons through Pyrolytic Dehydrogenation or Dehydrohalogenation
These methods produce fullerenes but not in sufficient quantities for practical applications. Rather, they provide a means of deducing the mechanisms of fullerene synthesis. For example, C60 consists of six dehydronaphthalene moieties located at the octahedral sites, and pyrolysis of naphthalene does indeed produce C60,7 as does corannulene, (which has been detected as a precursor in the combustion process), 7,10-bis(2,20 dibromovinyl)fluoranthene, and 11,12- benzofluoranthene. The dehydrogenation involved is a high-energy process and dehydrohalogenation of precursors is more successful, a feature made use of in formation of C60 from a chloroaromatic precursor. No other fullerene has yet been made in this way.
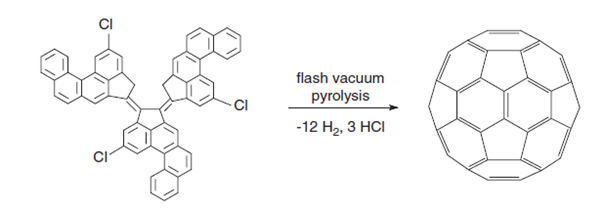
Figure 4: Formation of C60 fullerene through dehydrogenation/dehydrochlorination[4].
Applications
Although research on solid C60 fullerene is still at an early stage, these materials are already beginning to show many exceptional properties, some of which may lead to practical applications. Small-scale applications will come first; then targeted applications utilizing the special properties of fullerenes are expected to follow.
Optical Applications
- Optical Limiters
- Photoexcited C60-Polymer Composites
Electrical Applications
- C60 Transistors
- C60 Based Heterojunction Diodes
- C60 Polymer Composite Heterojunction Rectifying Diodes
- C60 Polymer Composite Heterojunction Photovoltaic Devices
- Photoresists
- Silicon Wafer Bonding
- Photolithography
Electrochemical Applications
- Hydrogen Storage and Primary Batteries
- Electrodes for Secondary Batteries
REFERENCES
1.E.W. Godly and R. Taylor, Pure Appl. Chem., 1997, 69, 1411; (b) E.W. Godly and R. Taylor, Fullerene Science and Technology, 1997, 5, 1667.
2.P.W. Fowler and D.E. Manolopoulos, Atlas of Fullerenes, Clarendon Press, Oxford, 1995.
3.L.D. Lamb, in The Chemistry of Fullerenes, R. Taylor (ed), World Scientific, Singapore, 1995, 20–30.
4.Hirsch, The Chemistry of the Fullerenes, Thieme, Stuttgart, 1994; F. Diederich, L. Isaacs and D. Philp, Chem. Soc. Rev., 1994, 23, 243; R. Taylor, The Chemistry of Fullerenes, World Scientific, Singapore, 1995; M.Prato, J. Mater. Chem., 1997, 7, 1097.
5.M.s. Dresselhaus-G. Dresselhaus-P.c. Eklund,Science of Fullerenes and Carbon Nanotubes, 1996.
REFERENCES FOR IMAGES
1.M.s. Dresselhaus-G. Dresselhaus-P.c. Eklund,Science of Fullerenes and Carbon Nanotubes, 1996.
2.M.s. Dresselhaus-G. Dresselhaus-P.c. Eklund,Science of Fullerenes and Carbon Nanotubes, 1996.
3.M.s. Dresselhaus-G. Dresselhaus-P.c. Eklund,Science of Fullerenes and Carbon Nanotubes, 1996.
4.M.s. Dresselhaus-G. Dresselhaus-P.c. Eklund,Science of Fullerenes and Carbon Nanotubes, 1996.
Silicon Carbide (SiC) Micron and Nano Powder
Silicon carbide nanoparticles have wide applications in field emission displays and light emitting devices. Moreover, nanoscale silicon carbide is used in designing and manufacturing electro-devices in nanoscale, nanosensors and biological labels. Bulky silicon carbide (SiC) is known as a refractory agent and semiconductor with excellent thermal, mechanical and chemical behavior. The melting point of this non-oxide ceramic is above 2800 °C, perfectly resistant against corrosion with low thermal expansion coefficient. Silicon carbide has a large bandgap and remarkable mechanical strength with low density and high hardness 1.
Silicon Carbide Properties
Silicon carbide, also known as carborundum, has a rare natural occurrence found in moissanite mineral. However, it has been largely produced as powder since the late nineteenth century with the most application as an abrasive agent. It been found applications in semiconducting electronics devices which function at high voltages and high temperatures. The most favorable electronic properties of silicon carbide include low quantum confinement, high ionic mobility, high breakdown electric field, and high saturation electron drift velocity and high thermal and electrical conductivity. Using a technique called Lely method, silicon carbide’s large single crystals are grown and cut as small as gems called synthetic moissanite. Through binding silicon carbide together, practically hard ceramics are used as a highly enduring agent in car clutch and ceramic plates, car brakes and bulletproof vests. Silicon carbide is also used in electronics, in designing detectors and light emitting diodes (LEDs). Even though silicon carbide is rarely found on Earth crust, it is highly abundant in space mostly near carbon-rich stars with the most common crystal forms as beta-polymorph. In general, crystalline silicon carbide comprises some 250 different forms and is produced in glassy amorphous crystals by pyrolysis of preceramic polymers in an inert gas atmosphere. The most common crystalline structure of SiC is alpha silicon carbide (α-SiC) with a hexagonal configuration which forms at temperatures over 1700˚C. There is a modified silicon carbide as β-SiC which is processed using a zinc blend crystal structure with applications in supports for heterogenous catalysts due to their higher surface area compared to the alpha form.
Read More About Silicon Carbide (SiC)
Silicon Micron and Nanoparticles powder
Particles and mostly crystals can be classified according to their size as nano and micron. Generally, the particles with their size around 70 microns are referred as micron particles while those with sizes below 100nm are considered as nanoparticles. Silicon carbide (Sic) is available as micron and nano scales with different properties as well. Regarding high fracture toughness, excellent hardness, high resistance towards oxidization, perfect mechanical strength and stability in extreme environmental conditions, silicon carbide is expected to adopt novel and even improved properties when its obtained in nano scale. The silicon nanoparticles have been demonstrated to exhibit new properties in addition to the improved behaviors mentioned earlier. Silicon nanoparticles have unique optical and electrical properties with higher strength and hardness when included in some target materials. Technically, the impact of silicon carbide nanoparticles has been investigated so precisely proving remarkable impact of the size their physical and mechanical behavior. It has been shown that the encapsulation of smaller silicon carbide nanoparticles in the bulk of aluminum has resulted in significant grain refinement when compared to SiC nanoparticle with bigger sizes. Moreover, the particle mean size has been demonstrated to influence the heat transfer capability and basic microscopic properties of α-SiC/water nanofluids. It has been proved that large-particle nanofluids have lower viscosity and higher thermal conductivity which is originated from the solid/liquid smaller area of interface. What’s more, the particle size directly and significantly affects the catalytic activity 2.
Synthesis and Preparation of Silicon Nano/Micron Particles and Powder
Numerous synthesis methods with different particle size based on the application has been reported in the literature. The methods introduced so far, have achieved particles in micron and nano size scales. However, the synthesis methods with nanoscale silicon carbides are mostly favorable. According to a method, silicon nanoparticles are prepared through using the wastes of compact discs (CDs) char as the carbon source silicon oxide for silicon and following the carbothermal reduction procedure at 1550˚C. the carbon from the CD wastes is extracted through pyrolysis technique in argon atmosphere at 700˚C for about 20 minutes. Silicon dioxide (SiO2) and the carbon extract are mixed based stochiometric ratio in a ring mill for a period of two hours to gain pellets. The pellets are put in a furnace to process the carbothermal reduction for an hour to finally obtain the silicon nanoparticles with the sizes ranging from 40 to 90 nm 1. Based on a report using the so-called sol-gel method, silicon carbide nanoparticles are synthesized using the tetraethyl orthosilicate (TEOS) as the precursor with hydrochloric acid and ethanol to control the pH and homogeneity of the reaction solution. According to this method, particle size shows to have increased the density and harness of resulting nanoparticles 3. In a technique, silicon carbide nanowires are prepared taking the advantage of metal catalyst free thermal evaporation technique. The process involves the placing silicon and graphite aside in a columnar graphite crucible with the entire system inside a vacuum furnace and argon gas atmosphere and heating it up to 1000C 4.

Applications of Silicon Nano/Micron Particles and Powder
Considering the mechanical strength, harness and thermal properties of silicon carbide with the improved quality in its nanoscale, silicon carbide nanoparticles have found applications in numerous sectors of industry. Silicon nanoparticles are famous for their practically wide bandgap in electronics and have been applied there. Another property that has made SiC nanoparticles so common and popular is their thermal conductivity. Also, in extreme environmental conditions, the use of a material with higher thermal conductivity seems essential. All the mentioned properties and specifications have led to silicon nano and micron particles application in electronic devices, field electron emission, sensors and power electronics. They are used as blue and ultra violet emitting diodes since they are capable of emitting sable and high-intensity blue and green light as well as ultra violet radiation. Silicon nano and micron particles have tuned out as effective and economical materials for industrial and engineering uses namely nanodevices, microelectronics, in biomedical engineering devices, nanocomposites, hydrophobic, and optoelectronics mainly due to the fact that SiC nanoparticles don’t need cooling down.
Click Image to Learn Also About Silicon
SiC carbides carbide nano and micron particles and powder show much improved and intensified properties compared to their bulky form. It has been shown that many properties of bulky silicon carbide are directly dependent on the particles size like the bandgap, as an example, has different widths when it is in bulky, micron and nano scales. SiC in its all particle size range show promising hardness, toughness with excellent mechanical strength.
References
1. Rajarao, R., Ferreira, R., Sadi, S. H. F., Khanna, R. & Sahajwalla, V. Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Mater. Lett. 120, 65–68 (2014).
2. Sun, B., Xie, R., Yu, C., Li, C. & Xu, H. Structural characterization of SiC nanoparticles. J. Semicond. 38, (2017).
3. Birru, A. K., Reddy, G. K., Ajay, G. & Kumar, N. K. Effect of Reinforced Silicon Carbide Nanoparticles in Epoxy Composites. Mater. Today Proc. 2, 4348–4352 (2015).
4. Mohd Sohor, M. A. H., Mustapha, M. & Chandra Kurnia, J. Silicon carbide- from synthesis to application: A review. MATEC Web Conf. 131, 1–6 (2017).
Molybdenum Oxide Nanoparticles
Nowadays, oxidation reactions play a substantial role in modern chemistry as well as chemical industry. Around 30% of total production in the chemical industry is carried out by the oxidation process. It means that after polymerization, oxidation is the largest process in the production of chemicals.
In the last few decades, transition metal oxides have attracted the research community in great deal due to their diversified applications. Among them, molybdenum oxide has been found to be one of the most fascinating materials due to its unique structural, optical, electrical, and mechanical properties and multidirectional applications such as gas sensing, photocatalysis, field emission (FE), capacitors, resistive switching, solar cells, light emitting diodes, hole transport materials, etc. Molybdenum is a Block D, Period 5 element, while oxygen is a Block P, Period 2 element in the periodic table. Molybdenum does not occur naturally as a free metal on EarthMolybdenum oxide is a wide bandgap material whose bandgap varies from insulating MoO3 (≈3.2 eV) to more conducting MoO3-x to semi-metallic MoO2 (≈2.55 eV). Molybdenum oxide exists in several phases such as MoO3 (orthorhombic, monoclinic, and hexagonal), Mo4O11 (monoclinic and orthorhombic), Mo5O14 (tetragonal), Mo8O23 (monoclinic), Mo9O26 (monoclinic), and MoO2 (monoclinic) depending upon the oxygen concentration and the oxidation state (+6, +5, +4, and +2) of Mo. The commonly used molybdenum oxide, MoO3, have three different polymorphs and these are:
i) a thermodynamically most stable orthorhombic structure(α-MoO3)
ii) a metastable monoclinic structure (β-MoO3) and
iii) a metastable hexagonal structure (h-MoO3).
Molybdenum oxide nanoparticle, also known as molybdenum trioxide nanoparticle, is a fine light blue powder composed primarily of MoO3 particles with diameters of 100nm or less generally.
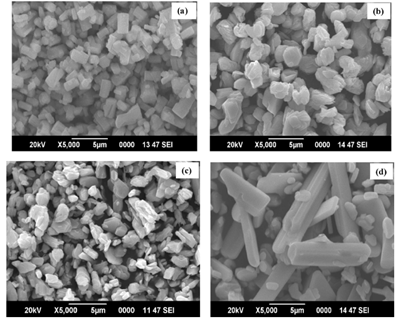
Figure 1: SEM image of MoO3 nanoparticles at different annealing temperatures (a) as-prepared, (b) 400 °C, (c) 500 °C and (d) 600 °C [1].
Due to high melting point, high chemical and thermal stability, and metallic conductivity, monoclinic MoO2 has attracted much attention in the research community.Looking to its high chemical stability, quick oxidation-reduction process, easy nanometric sized particles preparation procedure, we selected molybdenum oxides as viable candidates. Mo exists in three oxidation states and thus can readily participate in redox reactions and exhibits interesting metallic electrical conductivity. Hence, the nanostructured molybdenum oxide particles (MoOx NPs) could act as efficient electron redox mediators, contributing to the electrochemical sensing of some biological compounds such as dopamine. MoOx NPs are widely used in lithium ion batteries, capacitors and gas sensors, while their application in bio-sensing is still limited. Different types of MoO2 morphologies such as nanoparticles, hollow spheres, sheets, large scale nanowires, and nano stars have been synthesized via diversified techniques such as hydrothermal reaction, thermal evaporation, solution-based reactions, and hydrogenation processes. Molybdenum dioxide is also an unusual transition metal oxide because of its high metallic-like electrical conductivity, which is associated with its mixed interatomic bonding and a relatively high density of states at the Fermi level. The existence of free electrons in this region enhances the catalytic activity of Mo4+ in MoO2, unlike that of Mo6+ in MoO3, where all the valence electrons of the metal are covalently bonded to neighboring oxygen atoms. The metallic conductivity of MoO2 makes it a material of interest for many applications, including catalysis. Most recently it has been studied as a possible anode for lithium-ion batteries.
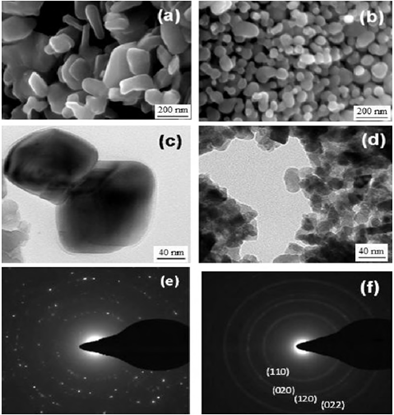
Figure 2: SEM and TEM images of commercially available MoO2 (a and c) and nanoparticle MoO2 (b and d). The corresponding selected area electron diffraction patterns asshown in (e) and (f), respectively. The index of electron diffraction pattern for nanoparticle MoO2 (f) matches to that of pure bulk MoO2[2].
Different morphologies of MoO3 nanostructures (NSs) such as nanoribbons, nanobelts, nanorods, nanowires, nanotubes, and nanosheets are prepared by various physical and chemical methods. Different techniques are deployed to synthesize MoO3 NSs successfully such as chemical vapor deposition (CVD), thermal evaporation, e-beam evaporation, pulsed laser deposition, sputtering, hydrothermal, molecular beam epitaxy (MBE), and van der Waals epitaxy.
MoO3 is a promising candidate to realize applications in electronic, photo-catalytic, gas sensor, biological activity, electrochromic, photochromic, lithium-ion batteries etc., owing to its tunable properties. It is an n-type wide bandgap semiconductor with three crystal structures (i) orthorhombic (α-MoO3) which is thermodynamically stable, (ii) metastable hexagonal (h-MoO3) and (iii) monoclinic (m-MoO3). Orthorhombic (α-MoO3) phase is widely studied by researchers because it gives out many applications. Molybdenum oxide nanoparticles can be synthesized through various methods like hydrothermal, microwave irradiation, co-precipitation, sono-chemical, flame synthesis, sol-gel etc. Among these methods, co-precipitation is a simple and cost-effective method capable producing large quantity of yield. There are many influencing synthesis parameters like acid or base, PH, surfactant, source material, annealing temperature etc., which could change the property of the bulk material significantly.
Laser Ablation of Solids in Liquids
The so-called LASL technique is an effective method to obtain nanostructures. This method is promising since the NPs formed can be free of both surfactants and other ions that exist during chemical synthesis, and it differs from laser ablation in vacuum or gaseous environments since the liquid can help to control some of the parameters of fabrication and to obtain the desired morphology and microstructure. In the LASL process, material is removed from the surface of a target in the form of plasma by the application of a high pulsed laser beam. Usually a target is submerged in a liquid and the laser is focused on the target through the transparent medium. When using the LASL method with metals, there are two main formation mechanisms proposed for the generation of nanostructures:
i) The thermal evaporation with liquid interaction, and
ii) The explosive ejection of nanodroplets. In the former, the formation of nanostructures is associated with the combination of ultrafast quenching of hot plasma and its interaction with surrounding media
In the latter case, it is suggested that the laser irradiation could cause a local melting from the metal target, the adjacent liquid layer is heated to vapor or plasma state with a high pressure, which splashes the molten target into nanodroplets that react with the liquid medium and create the final nanostructures.
Click Image to Learn Also About Molybdenum Sputtering Targets
Reduction of Molybdenum Trioxide Powder
Nanoparticle MoO2 is synthesized by reduction of molybdenum trioxide (MoO3) powder in a 1:3 volume ratio of ethylene glycol to distilled water. The mixture is combined in a Teflon-lined general-purpose vessel, which is subsequently sealed and heated to 180℃ for 12 hours. The liquid ratio of 1:3 was chosen because it yields pure single phase MoO2, without the need for any post-synthesis reduction. After cooling, the dark colored MoO2 was filtered and air dried at 100℃. It is relevant to note that the process used to produce the MoO2 nanoparticles is scalable and large quantities of catalyst could easily be prepared.
Applications
For molybdenum oxide nanoparticles, there are various types of applications. The key applications of molybdenum oxide nanoparticles are as follows:
- In electrochemical capacitors
- In coatings, nanowires, nanofibers, plastics, and textiles
- In specific alloy and catalyst applications
- As catalysts, oxidation catalysts, cracking catalysts, hydrogenation catalysts, and pigments
- In ceramics and glass production
- As a raw material for the production of molybdenum metal.
Catalyst applications: Like many nanomaterials, molybdenum oxide has several applications as a catalyst and is the subject of substantial research. Current known uses include hydrogenation catalysis and cracking catalysis.
Electrochemical applications: The unique electrical and chemical traits of molybdenum oxide at nanometer scales makes it of particular interest in the production of various displays and electrochemical devices.
Material production applications; Probably the primary current usage of molybdenum oxide lay in its versatility in the production of different materials. It serves as a seed material for producing various related substances, such as molybdenum metal, and is used as an additive and ingredient in various glasses, ceramics, and other compound materials.
Pigment applications: The unique optical properties of molybdenum oxide make it of occasional use as an additive for pigmentation.
REFERENCES
1.G. Pradeesh, V. Ponnuswamy, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties of molybdenum oxide nanoparticles prepared through chemicalprecipitation method for p-n junction diode application, Optics, 6 September 2018.
2.S. Muthamizh, R. Suresh, K. Giribabu, R. Manigandan, S. Praveen Kumar, S. Munusamy, A. Stephen, and V.Narayanan, Molybdenum oxide Nanotubes: Synthesis and characterizations, https://doi.org/10.1063/1.4917796 Published Online: 25 June 2015
3.J. Appl. Phys. 127, 025301 (2020); https://doi.org/10.1063/1.5127227 Submitted: 12 September 2019. Accepted: 21 December 2019. Published Online: 08 January 2020
4.G. Pradeesh, V. Ponnuswamy⁎, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties ofmolybdenum oxide nanoparticles prepared through chemical precipitation method for p-n junction diode application, Optics, 6 September 2018.
5.Le Xin Song, Mang Wang, Shu Zhen Pan, Jun Yang, Jie Chen and Jing Yang, Molybdenum oxide nanoparticles: preparation, characterization, and application in heterogeneous catalysis, Journal of Materials Chemistry, 24 March 2011.ssss
REFERENCES FOR IMAGES
1.G. Pradeesh, V. Ponnuswamy, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties of molybdenum oxide nanoparticles prepared through chemicalprecipitation method for p-n junction diode application, Optics, 6 September 2018.
2.Oscar Marin-Floresa, Timothy Turbab, Caleb Ellefsonb, Kang Wangb, Joe Breit c, Jeongmin Ahnb, M. Grant Nortonb, Su Haa, Nanoparticle molybdenum dioxide: A highly active catalyst for partial oxidation of aviation fuels, 2 June 2010.
Indium Tin Oxide
Indium Tin Oxide is a mixture of indium and tin. The typical composition by weight is 90% In2O3 and 10% SnO2. It is, therefore, indium oxide where a small fraction of indium atoms is replaced by tin atoms: we can say that it is indium oxide doped with tin. We will discuss all about it in this article.
Metals are of great importance in all areas of society, among them, Indium and Tin also stand out. Indium Tin Oxide has huge significance due to its unique properties which open the door for unlimited applications.
Let’s first find out about Indium and Tin individually:
Indium
The indium is an element of the periodic table. Its atomic number is 49 and its chemical symbol is In and its atomic weight is 114.82. It is relatively little abundant in the earth’s crust: approximately 0.000016% of the total materials in it correspond to this metal, which is equivalent to that for every kilogram of crust there are 0.25 milligrams of indium. Moreover, there are no known deposits where it exists in elemental form or in great concentration in any of its compounds; rather it is spread among other minerals. Therefore, the Indium obtained from the subsoil is a byproduct of the extraction of lead and zinc. Although in our times, the mining production of indium is lower in volume as compared to recycling products that contain it.
The Indium is so soft that it can be scratched with the nail. In its pure state, it is a soft and malleable material, silver in appearance; its density at 20 °C is 7.31 g/cm3. It even leaves a trace when rubbed on paper, as if it were a pencil. Its hardness is of value 1.2 on the Moh’s scale. In this gradation, the talc is assigned 0, while the diamond corresponds to 10; the wax has a hardness of 0.2, while 2.5 is the value for the material that forms our nails.
Indium is a metal that conducts electricity better than iron. Its electrical conductivity is of the order of 18% of the value corresponding to silver. Its boiling point is 2072 ° C and its melting point is 156.5985 °C. This value serves as one of the 14 calibration points of the international temperature scale (ITS-90). Also, due to its relatively accessible melting point, this material serves as a reference in the calibration of differential scanning calorimetry instruments.
Since an atom of indium has 49 electrons, its electronic configuration ends at level 5: 5s2 5p1. For this arrangement of electrons, its oxidation state is generally +3, although less often it usually has a +1 state. In the first state, it can form compounds with halogens such as InCl3, with oxygen (In2O3) and with sulfur (In2S3); the last two molecules only form at high temperatures. In the +1 state, it can form compounds such as InCl, InBr or In2O.
Now let’s find out about Tin:
Tin
Tin is known since ancient times and it was used mainly for the manufacture of weapons in times of war. Tin is a relatively rare element since it is not found in abundance on the planet. Its uses began around 3500 BC in the city of Ur, located in southern Mesopotamia, now known as Iraq. It began to be used in alloys with copper since when mixing copper and tin, they noticed a new metal they called bronze. This new material was resistant to corrosion due to the properties that tin grants to copper. During the development of this new material, an intense trade arose over long distances to obtain tin. The bronze turned out to be more efficient than the materials used at the time, Like rocks and bone. Today it is used to coat metals applied to food lateries, soft welding, label production, fungicides, pigments, dyes, among other applications.
Tin can be obtained from the mineral cassiterite or tin IV, being the most common mineral phase. It is obtained by reduction with coal. On the other hand, tin has taken great relevance when presented as a metal oxide, such as tin oxide (tin IV), because when mixed with the oxygen molecule, it can be used in applications such as chargers for lithium batteries, solar cells, optoelectronic devices, gas sensors, among others, where it encompasses electronic, optical and catalytic applications.
The SnO2 has a prohibited band of 3.6 eV and electronic mobility of 100 to 200 cm2 V-1 s-1. These values help us understand the ability of the material to apply in optoelectronics. It is considered an interesting material compared to other metal oxides due to its excellent stability, low cost, and its various applications.
Indium Tin Oxide Nanoparticles
Indium Tin Oxide Nanoparticles are of even greater importance in their nano size. It has a high transmittance, that is, it lets a high amount of energy pass to a medium. In addition to being transparent, it also has a low electrical resistivity, so it easily allows the movement of electric charge. All this occurs between visible and infrared radiation.
It is a highly degenerate n-type semiconductor (electrical conduction takes place by electrons). This material is weakly electronic conductor but remains transparent to visible light provided that it is in the form of a thin layer. This oxide is a better conductor of electricity than other transparent oxides like ZnO etc. The increase in electrical conductivity by doping leads to an increase in the absorption of light and the ITO layer turns yellow.
Indium Tin Oxide films on glass plates are obtained by different deposition techniques and generally by spraying (PVD for example).
Click Image to Read Also About Zinc Oxide
Applications of Indium Tin Oxide
1.Indium Tin Oxidefilms are mainly used as transparent electrodes for flat screen displays, touch screens, solar cells, organic light-emitting diodes (LEDs), certain anti-static coatings and screens against electromagnetic radiation but transparent to visible light. The development of electrochromic glazing is made possible thanks to this type of transparent electrodes deposited on glass. This type of deposit can also be used as a deicing system. Sheets of glass coated with transparent ITO films which conduct electricity are sold.
2.The most commonly used material for manufacturing conductive and transparent materials is tin and indium oxide.
3.The use of transparent conductive films from ITO is proposed as a new complete front electrode to replace conventional metal lines in the third generation group III-V solar cells.
4.The films were deposited by the DC-Magnetron Sputtering technique at room temperature and were optically, electrically and structurally characterized. Sample thicknesses were obtained by means of fixed wavelength ellipsometry. The crystalline structure of the deposited films was determined by x-ray diffraction, the diffractograms show the characteristic peaks of the typical bixite structure.
5.ITO films are used in cold regions and doors or walls of advanced buildings so that the heat stored in certain enclosed spaces. It plays a role in the thermal shield, and for the outside world, it is difficult to radiate heat to the room, due to which energy consumption in construction, heating, air conditioning, and lighting is reduced by more than 50%.
6.ITO thin film has an index of refraction and electrical conductivity, so it is suitable for a silicon solar cell and a collection of gross light antire reflector coating. Because it has a selective light-transmitting infrared light and reflectivity to visible light, the use of light-heat conversion and the effective use of solar heat can be made as to the permeable membrane, effectively trapping heat in the collecting reactor.
7.ITO is also used as a transparent film, a conductive function, such as an automobile coated glass, windshield machine, aircraft, and spacecraft laser porthole tank rangefinder.
8.This material is used to make transparent electrodes in display devices with flat screens (for example LCD screens) and electrochromic glazing.
9.Other uses include gas detectors, electro-moistening in dielectrics, and Bragg reflectors for vertical-cavity laser diodes emitting from the surface.
So, overall Indium Tin Oxide is used in the
- Preparation of sensitive surfaces
- Construction of lighting fixtures.
- Construction of electrical, electromagnetic and electronic apparatus and instruments.
- Construction of telephone, telegraphic, receiver-transmitter, electronic calculators.
- Construction of electrical systems.
- Construction of measuring and control instruments.
- Construction of cathode ray tubes, light bulbs, vacuum tubes or gas tubes.
- The inorganic and organic chemicals industry.
Smart Windows
Smart windows have a glass that selectively absorbs visible light when a voltage is applied, changing its degree of transparency to light and outside heat. These smart windows allow reducing the energy demand of a building (in lighting and air conditioning). Several professors from Univ of California in Berkeley published in Nature a new electrochromic material composed of indium and tin oxide (ITO) nanocrystals embedded in a glass of niobium oxide. One of the most interesting advantages of the new material is that it allows separate control of the light absorption in the visible and near-infrared, that is, in a smart window, optical and thermal transparency can be controlled separately and independently.
The performance of the new material is much better than expected, although there are some technical issues to be resolved before this new material can be used in windows in our homes (better electrode materials must be selected and it is convenient to use a solid electrolyte instead of liquid). Even so, it is a very promising job.
Researchers have developed a new synthesis technique to embed the nanoparticles in the glass. Indium and tin oxide (ITO) nanocrystals are coated with polyoxomethalates (POM), clusters of metal cations and oxo anions joined by covalent bonds to the surface of the ITO. These nanocrystals condense and form a film, which receives a heat treatment (heated to about 400 °C), and then densely packed in a niobium oxide (NbO) inside the glass matrix. Without going into technical details, this method provides a rigorous control of the size and spatial distribution of the nanocrystals in the glass. The latter is very important because the structural order of the nanocrystals greatly improves the properties electrochromic glass matrix.
Thus, Indium Tin Oxide is an important compound mainly used to make transparent and conductive coatings at the same time for screens. It is used in the making of liquid crystals, flat screens, plasma screens, touch screens, electronic ink applications, organic diodes, light-emitting diodes, photovoltaic cells, antistatic coatings, electromagnetic interference shields and much more.
References
https://www.researchgate.net/publication/322539063…
https://iopscience.iop.org/article/10.1088/1742-65…
https://aip.scitation.org/doi/10.1063/1.370948
https://www.researchgate.net/publication/234922459…
Lead Zirconate Titanate (PZT) Nanopowder
Lead zirconate titanate (PZT) and its nanoscale crystals are known as the most common ferroelectric inorganic compounds employed as piezoelectric materials with the highest coupling coefficient between its mechanical and electrical properties. Lead zirconate and titanate appears to have a morphotropic phase boundary at molar ratio fraction ranging from 0.4 to 0.6 supporting its sample processing setting and compositional homogeneity 1. The morphotropic phase boundary is a point where the Ti:Zr ratio is 48:52 at room temperature adequate to achieve the best piezoelectric properties. Lead zirconate titanateis originally a solution containing antiferroelectric PbZrO3 and ferroelectric PbTiO3 with their Currie temperatures as 230°C and 490°C, respectively 2. Generally, lead zirconate titanate shows better sensitivity at higher operation temperatures. Regarding these properties and chemical inertness and physical strength with relatively low preparation cost, lead zirconate titanate is among the most common piezoelectric materials in the industry.
Properties of Lead Zirconate Titanate Nanoparticles
Lead zirconate titanate nanoparticles properties are directly influenced by the doping sintered density and the structure of the materials based on PZT. Technically, the sintered density and structure depend critically on the process through which they are synthesized and the reagents used in for the synthesis. Considering the numerous studies concerning the piezoelectric, dielectric and optical properties of nanosized ferroelectric materials that have perovskite structure have drawn increasing attention. It is evident that materials in the nanoscale possess higher surface area to volume ratio compared to particles in micron and bigger scales and as a result, exhibit incredible and new chemical, physical, mechanical, optical and electrical characteristics. In this context, the lead zirconate titanate (PZT) nanoparticles Pb(ZrxTi1x)O3 are the subject of many studies because of their pyroelectric, dielectric energy storage capabilities, piezoelectric, electrochemical and electro-caloric effects, mechanical and electrical properties. In the case of lead zirconate titanate nanoparticles the best properties mentioned above are observed for compositions with their morphotropic phase boundary at near 0.5 3. In general, PZT nanoparticles and nanopowder (with relative permittivity between 300 to 20000) are in a wide range of frequencies with quick response time when used in detectors and could be applied in circuits with low or high voltages possessing good mechanical and acoustic coupling.

As a piezoelectric material, PZT adopts a potential difference under changing temperature conditions across its surface which physically changes shape when it is exposed to an external electrical field. PZT has a spontaneous electric polarization or electric dipole to be reserved in an electric field owing to its ferroelectric property. PZT is commercially not used alone and pure but is doped with either donor agents creating cationic vacancies as well as facilitating the domain wall motion in it or with acceptors creating anionic vacancies. The point is the acceptor doping results in harder PZT, whereas the donor doping causes softer crystals. Particularly, soft and hard lead zirconate titanate are different in their piezoelectric constant with the soft PZT crystals having a higher piezoelectric constant and the hard one with the lower piezoelectric constant. The reason the piezoelectric constant is proportional to the electrical field that is generated per unit of mechanical stress and polarization as well as the mechanical strain caused by the applied electric field.
Synthesis and Preparation of Lead Zirconate Titanate Nanoparticles
The significant roles that lead zirconate titanate nanoparticles have among the ferroelectric materials require the need for the best and most efficient methods with lowest expenses to obtain them in both in industrial and batch scales. Regarding this several synthesis methods have shown to possess enough quality to be applied for the PZT nanoparticles production. These methods and techniques involve co-precipitation, hydroxide co-precipitation, sol-gel combustion, hydrothermal, electro hydrodynamic atomization focused ion beam, microwave hydrothermal and electro-hydrodynamic atomization. In the sol-gel method, pure and homogenous PZT nanoscale particles are synthesized. The sol-gel preparation method takes place in a liquid solution of metal organic precursor leading to the formation of separate phase through the hydrolysis and condensation.
The main reagents to obtain ferroelectric lead zirconate titanate are Zr(OR)x, Ti(OR)x alkoxides and lead acetate Pb(OAc)2.3H2O which are made to react based on the sol-gel method. In so doing, a hetero-metallic alkoxide solution is prepared through mixing Pb, Zr and Ti and then stabilized by acetyl acetone. After the solution is prepared, an acidic or basic hydrolyzing agent is added to the solution to affect the pore structure and the stoichiometry of the gel. In this sol-gel method which takes the advantage of acetate-alkoxide, the amount of acetic acid as the solvent directly affects the particle size of lead zirconate titanate crystals, their homogeneity and stoichiometry 4. In a different method, lead zirconate titanate nanocrystals are synthesized using the citrate nitrate auto-combustion with the particle size ranging from 10 to 15 nm 2. One different sol-gel method of lead zirconate titanate nanoparticles preparation involves the synthesis at different pH values. Accordingly, the pH appear to affect transverse optical (TO) phonon modes, LO-TO splitting, the longitudinal optical (LO), extinction coefficient k(x) and refractive index n(x) 3.
Applications of Lead Zirconate Titanate Nanoparticles
The number research projects and publications submitted on the synthesis of PZT nanoparticles proves how capable and practical the nanoscale lead zirconate titanate are in science and industry. Therefore, the applications of PZT are broadly presented in the literature. The application of PZT nanoparticles originally arises from their piezoelectric and ferroelectric nature. They are employed in high temperature applications with truly high sensitivity in various sensors and actuators. Thin films of PZT are used in electronic and optical devices and memory devices. The medical applications of PZT are in ultrasonic transducers used for medical imaging. Moreover, they are used in underwater communication devices, electrical resonators, wave filters, fish finders, speakers and hydrophones. In addition to broad industrial applications, lead zirconate titanate nanoparticles have found optical applications in waveguides due to the PZT optical properties. In this category, the applications of lead zirconate titanate nanoparticles are in infrared (IR) optical field effect transistors, lamb wave devices and modulators. Considering the extensive range of infra-red spectroscopy, PZT nanoparticles are used in sensing, detecting, transmitting and pyroelectric IR detectors.
Lead zirconate titanate nanoparticles and nanopowder are inorganic ferroelectric agents with piezoelectric property and with the applications in piezoelectric resonators, ultrasonic transducers and IR spectroscopy. They are chemically inert and physically strong making PZT nanoparticles one of the most common piezoelectric compounds.
References
1. Garnica-Romo, M. G. et al. Nanoparticles of lead zirconate titanate (PZT) used as ferroelectric ceramics produced by sol–gel acetic-acid route. J. Sol-Gel Sci. Technol. 74, 425–431 (2015).
2. Banerjee, A. & Bose, S. Free-standing lead zirconate titanate nanoparticles: Low-temperature synthesis and densification. Chem. Mater. 16, 5610–5615 (2004).
3. Pakizeh, E. & Moradi, M. Effect of particle size on the optical properties of lead zirconate titanate nanopowders. J. Am. Ceram. Soc. 101, 5335–5345 (2018).
4. Garnica-Romo, M. G. et al. Nanoparticles of lead zirconate titanate (PZT) used as ferroelectric ceramics produced by sol–gel acetic-acid route. J. Sol-Gel Sci. Technol. 74, 425–431 (2015).
Nanomedicine and Nanodrugs
Nanotechnology has found practically extensive range of applications in almost all areas of science, technology and industry due to the surprising behavior, excellent performance and novel properties matter adopts when it falls into nano scale. Medicine has greatly taken advantages of nanotechnology not only in instrumentation but in drug synthesis with so many nanodrugs as well as applications in drug delivery and diagnostics. The findings based on nanoparticles and nanotechnology and the truly promising outcomes have led to an area in science called nanomedicine which specifically focuses on the application of nanotechnology and nanoparticles to promote the efficacy of medication, diagnostics and medicine at whole.
Click Image to Check A Similar Article on Blografi
Drug delivery systems based on nano technology are originally nanoparticles with one or more therapeutic agents that can bind to polymer matrices, encapsulated vesicles covalently or get dispersed or adsorbed in/on them. There has been substantial progress in the past 2 decades in the field of nanodrugs synthesis with products that are used in imaging, therapeutics and diagnostics. Basically, nanodrug systems focus on improved bioavailability of targeted delivery to particular tissues, prolonged half-life of injectable drugs (which is mainly done through reducing the immunogenicity) and orally administered medications. Totally, nanodrugs perform at lower quantities of administration with surprisingly improved and maximized pharmacological impacts as well as truly minimized health risks and side effects. The nanodrugs delivery systems comprise emulsions, solid lipid nanoparticles, micelles, dendrimers, nanocrystals liposomes and polymeric nanoparticles.
Patented Nanodrugs
So far, over 30 different nano-based drugs and treatments have officially been approved as patents by the U.S. Food and Drug Administration (FDA) and similar regulatory agencies across the world. The following sections mainly introduce and discuss the major patents in nanomedicine including Oxaliplatin, Octreotide-Modified Nanomedicine for Cancer Treatment, Nanoparticle Comprising Rapamycin and Albumin, Nanoparticles Comprising a Cholesteryl Ester Transfer Protein Inhibitor and a Nonionizable Polymer, Nanoparticles of Paclitaxel and Albumin in Combination with Bevacizumab against Cancer, Adenosine Deaminase anticancer therapy, Calcipotriol Monohydrate Nanocrystals
Click Image to Learn About Applications of Graphene in Medicine
Oxaliplatin- embedded nanoparticles are synthesized through lipid mixture and surfactant emulsifying in aqueous solutions. A particular co-solvent and Oxaliplatin are mixed in the solution. Oxaliplatin is a chemotherapy drug used in cholesterol cancer treatment 1. Octreotide-modified nanomedicine is a nanodrugs as small as 1 nm used in cancer therapy. This drugs mainly provides palliative care and cancer prevention like medullary thyroid carcinoma (MTC). Higher concentrations of octreotide-modified nanomedicine is effective for cytotoxicity against MTC cells. The preparation involves liposomes that are modified with octreotide which means the concentration of octreotide has to be kept low 2.
Click Image to Learn More About 8 Applications of Fullerenes in Medical Field
Nanoparticle containing Rapamycin and albumin used as an anticancer and cancer prevention agent. This patent involves a composition of nanoparticles that contain rapamycin and a protein carrier with application in cancer therapy 3. Nanoparticles serve to inhibit Cholesteryl Ester transfer protein and an Nonionizable polymer that is used to treat coronary artery disease 4. Nanoparticles of Paclitaxel and albumin in combination with Bevacizumab are used to treat cancer. The product is applied along with chemotherapeutic drugs with effective amount of taxane and a carrier protein in nanoparticles and an anti-VEGF antibody 5. Adenosine Deaminase is used in cancer therapy like treating tumors by administering a therapeutic dose of adenosine deaminase. This is a novel technique for tumor treating and inhibiting. Malignant solid tumors were discovered and recognized by administering adenosine deaminase 6. Calcipotriol monohydrate nanocrystals have application in preparing a tropical Calcipotriol that is capable of penetrating into skin with similar biological activities to Daivonex ointment. Calcipotriol is prepared as chemically stable nanocrystals 7. Liposomes containing docetaxel are prepared in the absence of heat, proteins, inorganic salts and organic solvents. This liposomal formulation has an application in treatment of diseases or disorders 8. Based on the use of silica nanoparticles with the particle size ranging from 1 to 100 nm, methods and Compositions have been developed for oral administration of Insulin. The pharmaceutical composition comprises a mixture of inert dry silica nanoparticles ingredients in an oil 9.
Nano Drugs in Ocular Drug Delivery
Ophthalmic drugs topical delivery has appeared to have a number of limitations with poor absorption as the most common one causing problems in the optimum quantity maintenance of the drug in the precorneal area of the eyes. The conventional topical ocular drugs have this limitation while based on the use of polymeric nanoparticles the stability of the drugs increases and they achieve a longer elimination half-life of 20 minutes in tear fluid. The nanodrugs used in this category have shown to promote the bioavailability, eliminate the side effects and enhance the absorption of the therapeutic drugs 10.
Nano Drugs for Central Nervous System Diseases
The presence and entry of any single molecule into the brain is basically organized by the blood-brain barrier (BBB) being regarded as a highly selective membrane barrier permeable for molecules with high o/w partition coefficient. Recently, nanoparticles have been applied to specifically serve as a drug carrier system at either side of this membrane. Particularly, nanoparticles go through the brain membranes based two main mechanisms namely their trans-synaptic transport through the olfactory epithelium followed by inhaling and crossing the BBB. Therefore, nanoparticles can be employed as a capable and efficient supporting agents to enhance the delivery of a given drug to brain 11.
Nano Drugs for the Treatment of Vascular Thrombosis
The common and conventional treatments for vascular thrombosis have very few benefits which generally lies on the short plasma half-life of drugs from the target sites, numerous side effects and rapid drug washout. These limitations could be overcome through immobilization of a particular amount of an agent onto a drug delivery system in order to enhance the stability and even the half-life of the encapsulated drug. Polymeric nanoparticles and liposomal nanocarriers are widely used due to biocompatibility and biodegradability.
Nanodrugs for Gene Therapy
There is a crystal clear fact that nanoparticles and nanotechnology at whole have considerably improved and promoted the conventional medical and pharmaceutical treatment strategies and quality. Gene therapy has also taken the advantages of nanodrugs and in this case, biological nanoparticles termed as bionanoparticles or viral vectors with diameters as small as 100 nm and less have shown to be stable and beneficial transgenes in cells. A lot of research has been focused on the application of viral vectors that are considered as drug delivery systems. Viral vectors as generally tools that are employed to deliver genetic materials and codes into a given cell. Among the common carriers in gene therapy, lentivirus, adenovirus, retrovirus and adeno-associated virus gene transfer have been investigated for use in cancer therapy.
The findings in nanomedicine and nanodrugs are so extensive and broad that can’t be listed and described just in one article. The fact is nanomedicine and nanodrugs have been and are causing remarkable changes in medicine and push it to a whole new level with the noticeable outcomes in healthcare. Nanonephrology, nanodrugs in cardiovascular disease treatment gene therapy and cancer therapy are regarded as the areas with great findings with a lot of ongoing research for better methods and techniques.
References
1. Kim, H., Heon, S., Kim, K. S., Application, F. & Data, P. ( 12 ) United States Patent — > CO , Flow High pressure. 2, (2016).
2. 0.pdf.
3. Soon-shiong, P., Angeles, L., Examiner, P., Hartley, M. G. & Sheets, D. ( 12 ) United States Patent. 2, (2014).
4. Bloom, C., Crew, M., Smithey, D., … W. M.-U. P. & 2014, undefined. Nanoparticles comprising a cholesteryl ester transfer protein inhibitor and anon-ionizable polymer. Google Patents 2, (2014).
5. Specification, E. P. TEPZZ Z97Z78B _ T. 1, 1–85 (2014).
6. Filpula, D., P Sapra – US Patent 8, 741,283 & 2014, undefined. Adenosine deaminase anticancer therapy. Google Patents 2, (2008).
7. Tepzz, T. Tepzz 5_5874b_t (11). 1, 1–27 (2014).
8. Panyam, J. & Chavanpatil, M. D. ( 12 ) Patent Application Publication ( 10 ) Pub . No .: US 2011 / 0020457 A1 Alginate core Patent Application Publication. 1, 0–5 (2011).
9. Pejaver, S. et al. ( 12 ) United States Patent. 2, (2006).
10. Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J. & Corrie, S. R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 33, 2373–2387 (2016).
11. Mukhopadhyay, S. Nano Drugs: A Critical Review of Their Patents and Market. A Critical Review of Their Patents and Market. Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery (Elsevier Inc., 2018). doi:10.1016/B978-0-12-814031-4.00018-0.
Copper Nanoparticles
Over the past few years, a considerable interest has been focused on metal nanoparticles due to their potential applications in diverse fields including catalysis, magnetic recording media, or microelectronics. The metal nanoparticles are finding applications in optoelectronics, nanodevices, nanoelectronics, nano-sensors, information storage and catalysis. Among various metal particles, copper nanoparticles have attracted more attention because of their catalytic, optical, electrical and antifungal/antibacterial applications. Copper nanoparticles are being researched a lot due to its high electrical conductivity, high melting point, low electrochemical migration behavior, excellent solderability and low material cost. Copper is one of the most widely used materials in the world. It has a great significance in all industries, particularly in the electrical industry due to its low cost.
Figure 1: TEM image of copper nanoparticles[1].
Production of Copper Nanoparticles
The synthesis of nanoparticles has received considerable attention because of their unique physical and chemical properties that are distinct from the bulk materials. Various methods are available for synthesis of copper nanoparticles. Copper nanoparticles received much attention because of large volume of literatures concerning the production and characterization of copper nanoparticles. Several methods have been proposed for synthesis of copper nanoparticles by considering bottom-up and top-down methods. The major techniques for preparing copper nanoparticles by chemical methods are chemical reduction, sonochemical reduction, micro emulsion techniques, electrochemical, hydrothermal, sol gel synthesis, polyol process, biological synthesis and microwave assisted techniques. Physical methods for the synthesis of copper nanoparticles are laser ablation, vapor phase synthesis, mechanical milling and pulsed wire discharge. Chemical methods of preparing copper nanoparticles are simple in operation, high exile and environment friendly. In electrochemical method, the copper nanoparticles can be produced at room temperature without using any additives. The main advantages of powder production by electrolysis are high purity and there is no need to use high pressure, toxic chemicals and vacuum systems to produce the product.
Click Image to Learn More About Top-Down Bottom-Up Approach
Copper nanoparticles can be produced by using various techniques typically classified as bottom-up or chemical methods and top-down or physical methodswhich is shown in Fig. 1. The structure of copper nanoparticles is composed of atoms and clusters or molecules in bottom-up approach where as in the case of top-down approach a bulk piece of required material diminished to nanoscale dimensions using mechanical milling, cutting, etching and grinding techniques. Some of the methods like chemical reduction,sonochemical reduction, microemulsion techniques,electrochemical,hydrothermal, sol–gel synthesis,polyol process and microwave-assisted methodsare the major techniques for preparing copper nanoparticles from bottom-up approach. Biological syntheses are also treated as bottom-up approach.Top down methods for synthesis of copper nanoparticles are laser ablation,vapor phase synthesis,mechanical millingand pulsed wire discharge (PWD).
Figure 2: Classification of synthesis techniques of copper nanoparticles[2].
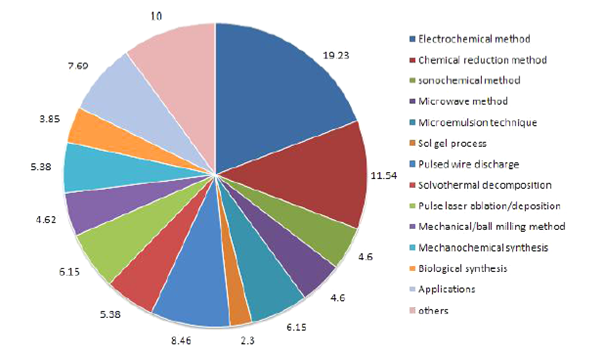
Figure 3: Pie chart for synthesis of copper nanoparticles[3].
Applications
Copper nanoparticles have received considerable attention due to their physicochemical properties such as high melting point temperature, magnetism, electrical and thermal conductivity, light absorption and high heat transfer. Copper nanoparticles are used as an antimicrobial agent due to their high surface-to-volume ratio and easy interaction with other particles to enhance their antimicrobial efficiency. Copper nanoparticles are highly reactive compared to other metallic nanoparticles. Due to these special properties and small dimensions, copper nanoparticles hold important application in heat transfer systems, sensors, high strength materials, catalysts, antimicrobial materials, etc.
The usage of biodiesel in automotive engines is limited due to its low performance and high nitrogen oxide emission. Nano-copper particles was used as the fuel additive for diesel engine with Soya bean biodiesel (B10). The copper nanoparticles were prepared using electrochemical method and the size of Cu nanoparticles were in the range of 40–50 nm. Nano-copper particles exhibits better engine performance and reduced nitrogen oxide emission when compared to other formulations.
Anti-microbial effects of metal nanoparticles received considerable attention from both medical and technological point of view. Metallic Cu particles are used as an anti-infective agent for replacing Ag and other noble metal composites and finds applications such as water treatment, food processing and protection of medical instruments. Glucosamine-functionalized copper nanoparticles synthesized from injection pump-assisted reaction environment and in situ reflux condensation exhibits outstanding anti-bacterial activity against two Gram-negative and Gram-positive strain. The dynamic and structural light scattering reveals that glucosamine functionalized copper nanoparticles are spherical in shape with hybrid morphology.
Metallic copper nanoparticles and its composites provide flexible interface activity in biological and biosensing applications due its special properties like bio compatibility, high stability against aggregation and hydrophilic character. Glucosaminesurface-modified copper nanoparticles prepared by integration of injection pump and ultra-sonochemistry.
Click Image to Learn About Copper
Even though bulk microstructures and the nanostructure of metallic copper demonstrate distinct applications, the functionalized copper nanoparticles exhibit advanced features such as high thermal stability, electrochemical feasibility toward biomolecule (C-reactive protein, CRP) detection and more crystallinity. The development of miniaturized nanodevices that incorporates electronic, chemical, photonic and biological features is essential for future electronic and sensing devices. The photosensitivity and extensive cross section of metal nanoparticles makes them highly applicable in several applications such as optical sensors and in surface enhanced spectroscopes. Cu nanoparticles prepared by nanosphere lithography may potentially be used for replacing expensive metals of Ag and Au in certain applications.
Electrochemical capacitors are received considerable attention because of their long-life cycle, high power density and excellent reversibility. Copper oxide multilayer nanosheets prepared by chemical bath deposition method shows hydrophilic nature with water and the copper oxide electrode exhibits super capacitance. This shows copper oxide thin films are most suitable electrode material for electrochemical capacitors. Anti-microbial effects of metal nanoparticles received considerable attention from both medical and technological point of view. Metallic Cu particles was used as an anti-infective agent for replacing Ag and other noble metal composites and finds applications such as water treatment, food processing and protection of medical instruments. Glucosamine-functionalized copper nanoparticles synthesized from injection pump-assisted reaction environment and in situ reflux condensation exhibits outstanding anti-bacterial activity against two Gram-negative and Gram-positive strain. The dynamic and structural light scattering reveals that glucosamine functionalized copper nanoparticles are spherical in shape with hybrid morphology.
Metallic copper nanoparticles and its composites provide flexible interface activity in biological and biosensing applications due its special properties like bio compatibility, high stability against aggregation and hydrophilic character. Glucosaminesurface-modified copper nanoparticles prepared by integration of injection pump and ultrasonochemistry. Even though bulk microstructures and the nanostructure of metallic copper demonstrate distinct applications, the functionalized copper nanoparticles exhibit advanced features such as high thermal stability, electrochemical feasibility toward biomolecule (C-reactive protein, CRP) detection and more crystallinity.
The development of miniaturized nanodevices that incorporates electronic, chemical, photonic and biological features is essential for future electronic and sensing devices. The photosensitivity and extensive cross section of metal nanoparticles makes them highly applicable in several applications such as optical sensors and in surface enhanced spectroscopes.
Cu nanoparticles prepared by nanosphere lithography may potentially be used for replacing expensive metals of Ag and Au in certain applications. Electrochemical capacitors are received considerable attention because of their long-life cycle, high power density and excellent reversibility. Copper oxide multilayer nanosheets prepared by chemical bath deposition method shows hydrophilic nature with water and the copper oxide electrode exhibits super capacitance. This shows copper oxide thin films are most suitable electrode material for electrochemical capacitors.
Physical method of preparing nanoparticles requires expensive equipment, high temperature and vacuum systems. Biological methods are also used for synthesis of copper nanoparticles, but due to lack of knowledge and experience it is not applicable to produce nanoparticles. Chemical methods are employed for synthesis of nanoparticles due to its low cost, simple operation, high edibility, easy availability of equipment, no need of vacuum systems, environment friendly and provides high yield in ambient conditions.
In a general way, it can be concluded that the formation of Cu-NPs is extremely sensitive to the synthesis conditions. Therefore, before using a synthesis method described in the literature, one should observe the real influence of synthesis conditions such as temperature, stirring and composition of the mixture for an effective control of size distribution and particle shape. Also, it should be considered that the metallic nanoparticles are rather a new research subject in science world. Although cobalt nanoparticles have some outstanding properties, it is a rather new material and more research should be done in order to enhance the properties and moderate the disadvantages. A lot of researches are still being conducted to improve its properties and lower its manufacturing cost in order to implement its use in real life applications. In the coming years, this material will innovate the world of technology due to its unique characteristics.
REFERENCES
1.P.K. Khanna, S. Gaikwad, P.V. Adhyapak, N. Singh, R. Marimuthu, Synthesis and characterization of copper nanoparticles, Materials Letters, 13 March 2007.
2.Jeyaraman Ramyadevi, Kadarkaraithangam Jeyasubramanian, Arumugam Marikani,
Govindasamy Rajakumar, Abdul Abdul Rahuman, Synthesis and antimicrobial activity of copper nanoparticles, Materials Letters, 19 December 2011.
3.N. Arul Dhas, C. Paul Raj, and A. Gedanken, Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles, Department of Chemistry, Bar-Ilan University, 25 February 1988.
4.A. Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
5.R. Bali, N. Razak, A. Lumb and A. T. Harris, The synthesis of metallic nanoparticles inside live plants. Int. Conference on Nanoscience and Nanotechnology, 2006. ICONN ’06., Brisbane, Qld, (2006).
6.Yanhu Wei, Steven Chen, Bartlomiej Kowalczyk, Sabil Huda, Timothy P. Gray, and Bartosz A. Grzybowski, Synthesis of Stable, Low-Dispersity Copper Nanoparticles and Nanorods and Their Antifungal and Catalytic Properties,Department of Chemical and Biological Engineering, Department of Chemistry, Northwestern UniVersity, 31 July 2010.
REFERENCES FOR IMAGES
1.N. Arul Dhas, C. Paul Raj, and A. Gedanken, Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles, Department of Chemistry, Bar-Ilan University, 25 February 1988.
2.Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
3.Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
Aluminum Nitride (AIN) Nanoparticles
Aluminum nitride is a compound having remarkable characteristics such as high thermal conductivity. Due to these characteristics, it has a wide range of applications in the electronics sector. The thermal conductivity of Aluminum nitride is in the range of 170-180 W/mK. This is greater than a lot of other ceramics. Moreover, Aluminum nitride can be easily metalized and it is considered a good electric insulator. This characteristic makes it possible to use Aluminum nitride as a heat sink or where the quick heat removal is desired. Aluminum nitride can be found in large shapes, thin substrate, or in powder form. The characteristics of Aluminum nitride are even more effective in the form of nanoparticles. Therefore, in this article, we aim to discuss the properties, applications, and potential of Aluminum nitride nanoparticles for future use.
Introduction
Aluminum nitride has exceptional chemical resistance and thermal conductivity. AlN is a wide-bandgap semiconductor (6.2 eV). It is a refractory and electrically insulating material having a very high thermal conductivity (higher than that of copper at 200 °C) and having a high resistance to oxidation and abrasion. It has potential applications as a substrate and in power electronics for the manufacture of microwave power transistors. Aluminum nitride is synthesized by the thermal reduction of alumina. It is transparent to visible wavelengths and infrared (0.5 to 3 μm) and can be used as a window for infrared and radars.
But let’s first find out about Aluminum to develop a better understanding of Aluminum Nitride:
About Aluminum
Aluminum is a soft and silvery-white metal with atomic number 13. It has remarkable characteristics. The fusibility (a term used to refer to the melting temperature) of aluminum is 660˚C. Its toughness can be modified by alloys, but in general, it is significantly lower than iron. The comparison of its lightness with glass is confusing. Depending on the composition of the glass, whose density can vary from about 2 to 8 tons per cubic meter, the density of aluminum is approximately 2.7 tons per cubic meter, which is much lower than that of iron (7.8 ton/m3).
The low density of aluminum in relation to iron (just about 3 times less) is undoubtedly one of the most attractive properties of aluminum. The possibility of having metallic instruments much lighter than the existing ones was what led several researchers to seek improvements in the Deville process. At the end of the 19th century, Paul Heroult (French) and Charles Martin Hall (American) independently developed a process to efficiently extract metallic aluminum from the most common oxide, alumina. Both researchers mutually recognized each other’s originality and, to date, the process is known as Hall-Heroult and is still widely used to produce metallic aluminum. The Hall-Heroult process was complemented by that of Karl-Josef Bayer (son of the founder of the famous pharmaceutical company) who developed an economical way to produce alumina from the most abundant aluminum ore: bauxite. Thus, at the beginning of the twentieth century, aluminum made its triumphant arrival in the global context of metallic materials.
The previous sentence is not a hyperbole. By the middle of the 20th century, aluminum alloys were the second most used in the world, only below ferrous ones. Its low density allowed aluminum to be used in multiple applications where low weight metal elements were needed. And among those applications, the manufacture of transport vehicles is the most important. This was recognized by the Wright brothers, who in 1903 ordered the crankcase (crankshaft support) of their famous aircraft to a local aluminum smelting company. This fact happens to the second term before the relevance of the historical flight, but it meant that aluminum could be used successfully in engine parts. In 1957, the Soviet Union launched Sputnik 1.
At present, as with all metals, the properties of aluminum are improved through alloys. It is possible to empty it in complex shapes and thanks to its crystalline structure, it can withstand large deformations. The applications of aluminum alloys range from the fuselage and aircraft wings (often reinforced with carbon fibers), multiple car parts (such as engine heads, pistons, and wheels), ladders and scaffolding, to beverage cans and ultrathin sheets commonly known as the foil. In Mexico, the manufacture of aluminum alloy products is one of the most important processing industries. According to 2017 data, the production of aluminum parts reports profits of about seven billion dollars annually.
Aluminum has many important compounds such as Aluminum Nitride. Let’s have a look at the characteristics of Aluminum Nitride.
Click Images to Read About Aluminum Oxide Nanoparticles
Properties of Aluminum Nitride
The properties of ceramic aluminum nitride are unique as it combines high electrical resistivity with high thermal conductivity. It is to be noted that not all ceramics possess high thermal conductivity. Only Cubic boron nitride, beryllium oxide, and aluminum nitride possess this characteristic. But, beryllium oxide cannot be used due to its high toxicity. On the other hand, cubic boron nitride is very complicated to manufacture. Thus, only aluminum nitride is an effective compound to use in applications where high thermal conductivity is required.
Thermal Conductivity is defined as the capacity of a component to conduct heat due to temperature difference. Heat is conducted in Aluminum Nitride via reticular vibrations, which is also termed as “phonons”. It has been noted that materials having low atomic mass, simple structure and covalent bonds possess high thermal conductivity.
Several factors affect the thermal conductivity of the material by hindering the transmission of phonons. These factors include Temperature, pore size and distribution, impurities, grain size, orientation, and homogeneity of the composition. They affect network vibrations which hinder thermal conductivity.
AIN has very high thermal conductivity, up to 220 W. It is around ten times higher as compared to alumina. The theoretical value of thermal conductivity for aluminum nitride is around 280 W/mK. The actual value of thermal conductivity for aluminum nitride is dependent on the purity of the raw material and the processing conditions.
The impurities of oxygen are a significant detriment in this regard because it replaces nitrogen in the structure of aluminum nitride and leaves spaces that hinder the transmission of phonons and disperse them which in turn affects the thermal conductivity of aluminum nitride. AIN devices have high hardness, high modulus, very high dielectric properties, good oxidation-resistant property and efficient low-thermal expansion, which is approximate to of silicon. When using AlN’s powers to make compounds, its interface compatibility is good. It can improve thermal conductance, mechanical properties, and dielectric properties of compounds.
In short, the main characteristics of aluminum nitride powder:
1.Excellent thermal conductivity
2.High operating temperature
3.Low coefficient of thermal expansion
4.Low dielectric constant
5.Good electrical insulation and electrical resistivity
6.Mechanical resistance in compression
7.The corrosion resistance of gases
8.High thermal conduction, 5 times that of alumina
9.Resists molten metals (aluminum, copper, lithium, etc.)
10.High purity
11.Attacked by acids and alkalis
Applications of Aluminum Nitride Nanoparticles
Aluminum Nitride Nanoparticles are widely used in the ceramic substrate.
Aluminum nitride ceramic (AIN) with high thermal conductivity, low coefficient of thermal expansion, high strength and other characteristics, as well as good mechanical properties, is considered the next generation of high-performance ceramic substrate and packaging material of choice.
Aluminum nitride ceramic (AIN) has two very important properties that are worth highlighting: a high thermal conductivity and an expansion coefficient of whether they are combined. The disadvantage is that even on a very thin oxide surface, the oxide layer will have an impact on the thermal conductivity of the materials and will process only strict control to create a better substrate consistency. AlN has still immaturity of large-scale domestic production technology, relative to Al2O3, since many relatively high prices, this is a bottleneck that restricts its development but researchers are consistently working to overcome this aspect.
AIN ceramics have a high thermal performance for the high power semiconductor substrate, the natural heat cooling process to achieve its objectives, but also has good mechanical strength, excellent electrical properties. There is a need to improve domestic manufacturing technology, and the price is more expensive, but its annual growth rate is more than 4 times to ceramics, beryllium oxide, and later can be replaced by a series of non-rust ceramics.
The other main uses of aluminum nitride (AIN) nanoparticles are:
1.Heat sink materials for power transistors, thyristors, LDs and LED
2.Electronic components where electrical dissipation of insulation and heat is required
3.Circuit substrates of semiconductor modules and IC packages
4.Crucibles for molten metals, vacuum deposition, and single crystals
5.Window materials for infrared and radar applications
6.Heat sink for uncooled power laser diodes
7.Components where a low dielectric constant and a low dissipation factor are required
8.Fasteners where a low coefficient of thermal expansion is required
9.Electrical insulator for electronic tubes
10.Electrical insulator for lasers
11.Chip holder for sensors and detectors
12.Electrical insulator (chuck, clamp ring) for semiconductor process machines
13.Substrate and insulator for microelectronic circuits, for optoelectronic systems
Research
Researchers from the Higher Council for Scientific Research (CSIC) have developed supports for low-energy LED bulbs that dissipate heat better. The development is based on the stacking of different multilayer substrates of aluminum nitride (AIN), a material with high thermal connectivity.
The manufacturing method of these supports will be transferred to a Russian company interested in its industrial development. A team from the Institute of Ceramics and Glass of the CSIC has developed the methods to tape the substrates and incorporate the tungsten circuits by screen printing. In addition, it has carried out the stacking processes of the different layers and heating them at a temperature of 1,900 °C so that they are perfectly integrated into the system.
These LED bulbs are characterized by much lower power consumption, longer life, smaller size, durability and resistance to vibrations. According to the Researchers, “Aluminum nitride is a material that dissipates heat very well and is ideal to withstand high power densities, which generate a lot of heat, as in the case of LEDs. Ceramic brackets of this type help to cool, further prolonging the durability of the bulbs”. The research is of high importance for the development of other ceramic products with high added value, such as biomaterials used in bone restoration or ceramic shields.
In conclusion, Aluminum nitride (AlN) is of great importance particularly due to its high thermal conductivity which is about 5-10 times as of alumina ceramic. Moreover, it has a low dielectric constant and dissipation factor, outstanding mechanical properties, good insulation, high heat resistance, non-toxic, chemical resistance, and the linear expansion coefficient is the same as of Silicon. Aluminum nitride (AlN) is extensively being used in the electronics industry and in the form of nanoparticles; Aluminum nitride (AlN) is unleashing more interesting applications.
References
https://www.azom.com/article.aspx?ArticleID=4463
https://www.azonano.com/article.aspx?ArticleID=3317
https://www.sciencedirect.com/topics/chemical-engineering/aluminum-nitride
https://www.sciencedirect.com/topics/materials-science/aluminum-alloys
Gold Nanoparticles Against Pathogens of Coronavirus Family
Coronavirus (2019-nCoV) researches continue to find a remedy for the spread. All disciplines are working in a coordination to stop this disease. There are test, vaccine, and treatment researches all around world while time is ticking. However, answer might be in nanotechnology to find COVID-19 test, or to develop a vaccine for coronavirus. Below, we dived into the possible scenario of using gold nanoparticle to fight against coronavirus family and other nanotechnological contributions to halt novel Coronavirus.
Coronavirus pathogens including SARS-CoV that stands for sever acute respiratory syndrome coronavirus, SARS-CoV-2 which is responsible for coronavirus disease 2019 (COVID-19) and Middle East respiratory syndrome (MERS-CoV) cause life-threatening pneumonia in humans with almost no licensed and specific therapeutic vaccines so far. In the case of COVID-19, which is the most contagious and deadliest coronavirus disease with nearly two million infected people worldwide and over 140 thousand deaths, the 70-yearl-old malaria drug, chloroquine has shown to have some confirmed therapeutic effects, lately. However, some clinical reports have confirmed that a quarter of patients with COVID-19 have developed heart disease. Moreover, several vaccines which take the advantage of nanoparticles have been introduced to fight MERS-CoV. Even though, there are few antiviral drugs and vaccines for therapeutic purposes, poor metabolic stability, serious adverse health effects and low antiviral activities have greatly impaired their remedial effects 1. Consequently, there is an urgent need to develop practical and effective treatment for the newly arisen COVID-19.
Click Image to Reach Our Other Articles About COVID-19
Coronavirus Pathogens Binding and Fusion Mechanism
Prior to any mechanistic discussion over the pathogens of coronavirus family, it is helpful to review the molecular structure and types of protein they consist of. Coronaviruses are a family of enveloped positive-stranded RNA viruses comprising 26 to 32 kilobase genome which is regarded as the largest among the RNA of all viruses. They are found among an extensive range of hosts capable of infecting species like mammalians and birds as well as serious health disorders such as gastrointestinal, respiratory, hepatic and central nervous system diseases. In coronaviruses, the spike protein forms crown-like (Corona in Latin) structures coming out of the virus surface used to penetrate the host cells. The spike protein itself comprises S1 and S2 where the S1 binds to host cell receptor and S2 for membrane fusion. The nucleocapsid protein is considered as the most abundant protein in coronavirus and a highly immunogenic phosphoprotein. It should be noted that the nucleocapsid protein hardly ever does mutate and is used as a marker in diagnostic assays 2.
The mechanism through which coronavirus pathogens bind to cellular receptors with a consequent membrane fusion mediation is the use of the spike (S) protein. An increasing amount of studies and experiments demonstrate the S protein involvement in the infection. The S1 proteins as it was introduced above, binds to host cells through dipeptidyl peptidase 4 receptors (DPP4) with the consecutive triggering of S2 protein in order to mediate the fusion of the virus envelope with host cell membrane. The S2 protein’s censorious role to regulate the infection by coronaviruses demonstrates its inhibition function is a powerful technique in treatment. Specifically, the S2 protein is made of three main areas including heptad repeat 1 (HR1), heptad repeat 2 (HR2) and fusion peptides (FP). After the S1 proteins binding to the DPP4 is done, the fusion peptide goes through the membrane of the host cells. Later, the HR1 and HR2 twist around each other forming a helical structure called 6-helix bundle (6-HB) which itself is responsible for drawing the host cell membrane and the envelope of the virus toward each other and promotes their fusion. This fusion results in the release of the viral genome (RNA) into the host cell. According to this mechanism, 6-HB formation seems to be a critical factor in mediating the coronavirus fusion with cell membranes 1.
Click Image to Find Out More About Nanomaterials in Medicine
Currently, the most possible strategy against the spike protein is employing antibodies that are capable of blocking the virus binding and fusion in order to neutralize the infection. As a result, the spike (S) protein has to be regarded as a drug or vaccine target candidate to suppress the infectious process and replications. Studies and experiments conducted on animals suggest that any inadequate protective immune system against the pathogens of coronavirus can lead to an eosinophilic immunopathology in the lungs when the body is infected 3.
Since the SARS-CoV-2 is a newly emerged pathogen from the coronavirus family and there are very few treatments based on nanotechnology, the upcoming section will specifically focus on the previously developed gold nanoparticle-based treatment methods against SARS-CoV and MERS-CoV. However, a precise review of the past studies can help researchers come up with novel methods to specifically deal with the pandemic COVID-19 today that has claimed thousands of lives across the world.
Gold Nanoparticles for Detection and Inhibition of Coronavirus Pathogens
Colorimetric techniques have shown to serve as effective tools to detect and identify target analytes through the change in the color of an indicator such as a metallic nanoparticle and fluorescent molecules. The detection involves approving a biomarkers attendance or presence based on the visual observation through measuring the absorbance of the dye agent at a particular characteristic wavelength. Based on a recent study carried out in early 2019, a colorimetric assay as a detection platform was developed to detect MERS-CoV using double-strand DNA (dsDNA) self-assembly coated or shielded with gold nanoparticles in magnesium chloride as a positive electrolyte. This platform is capable of detecting MERS-CoV by the change in the color gold nanoparticles in the ultra violet and visible (UV-Visible) wavelength range and the shift in their local surface Plasmon resonance (LSPR). Accordingly, a pair of probes at the 5′ end or 3′ end of the double-stranded DNA is designed and modified with thiol in order to organize complementary base pair with upstream of the E protein gene and open reading frames (ORF) on MERS-CoV. In this order, the probes and the targets’ dsDNA form a disulfide-induced long self-assembled complex to protect the gold nanoparticles from transition of optical properties and self-induced aggregation. This colorimetric detection platform can discriminate 1 to Pico molar per one micro liter (1 pM/µL) of MERS-CoV 4. Regarding the similarities in structure, this platform can potentially be employed to develop SARS-CoV-2 kits with some modification.
Gold Nanoparticle-Modified Spike Protein as a Potent Antigen against Coronavirus
Gold nanoparticles, which are believed to act as an adjuvant and an antigen carrier in immunization simultaneously, are considered as an effective adjuvant in SARS-CoV vaccine which is inactivated by ultraviolet radiation. In this method, the preliminary tests were conducted on mice through immunizing them by 0.5 µg spike protein (S) in the absence of adjuvant SARS after the infection is done with mouse-adapted virus. The protein that is adjuvanted with gold nanoparticles lead to a potent IgG response but fails to increase the efficacy of the vaccine or stop/decrease the eosinophilic infiltration. Based on the results of this study, gold nanoparticle‐adjuvanted S protein can result in a promoted antigen-specific IgG response against SARS-CoV 3.
Figure 1. Schematic Diagram of Coronavirus
HR1 Peptide Inhibitor Based on Gold Nanorod
Taking the advantage of gold nanorods in this method, a set of inhibitors of heptad repeat 1 (HR1) has been developed in order to prevent HR1/HR2-mediated membrane fusion between the host cells and MERS-CoV. Specifically in this technique, peptide pregnancy-induced hypertension (PIH), which has turned out to show strong inhibition against MERS-CoV, becomes even 10 times stronger when it forms a complex with gold nanorods (PIH-AuNRs) exhibiting an additional promoted biocompatibility and metabolic stability. This complexation has shown a novel antiviral activity with a potential quality to be used in developing clinical forms as drugs or vaccine in treating MERS-CoV infection 1.
Conclusion
With regard to the structural similarities among the pathogens of coronavirus including MERS-CoV, SARS-CoV and the novel SARS-CoV-2, gold nanoparticles can not only serve as treatment strategy against COVID-19, but they can be applied to design coronavirus diagnostic kits. As the previous studies have proved the satisfactory results gold nanoparticles applications, studies concerning developing AuNP-based platforms can emerge as a relief in the critical moments the world has gone through.
References
1. Huang, X. et al. Novel Gold Nanorod-Based HR1 Peptide Inhibitor for Middle East Respiratory Syndrome Coronavirus. ACS Appl. Mater. Interfaces 11, 19799–19807 (2019).
2. Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 3, 237–261 (2016).
3. Sekimukai, H. et al. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 64, 33–51 (2020).
4. Kim, H. et al. Development of Label-Free Colorimetric Assay for MERS-CoV Using Gold Nanoparticles. ACS Sensors 4, 1306–1312 (2019).
SiO2 Nano powder, Properties & Applications
Silicon dioxide (SiO2) is a compound of Silicon and Oxygen, commonly called silica and the elements are linked by the covalent bond. It is one of the components of the sand and can be found naturally in Quartz. It is usually white or colorless and is not soluble in water or ethanol. By associating with minerals, it forms the silicate family. Silicon dioxide (SiO2) has several industrial applications such as an additive in the food industry. Its function is to act as an anti-binder, anti-foaming agent, viscosity controller, desiccant, beverage clarifier and as an excipient of medications and vitamins. Due to its insolubility in water, silica has little biological availability and is not considered a source of silicon. Silicon found in other more soluble forms contributes to the formation and maintenance of bones and cartilage.
In this article, we will review the properties and applications of SiO2 Nanoparticles in multiple sectors:
Let’s start with its properties:
Properties of Silicon dioxide (SiO2) Nano Powder
Silicon dioxide is a solid and colorless crystalline substance. Silicon dioxide does not react with water and is resistant to acids. The molecular formula of the substance is SiO2. Silicon oxide, a member of the group of acidic glass-forming oxides, interacts with increasing temperature with alkalis and basic oxides, soluble in hydrofluoric acid, tends to form a supersonic fusion, that is, glass is an excellent dielectric. The main properties are stated below:
- SiO2 Nanoparticles is transparent and its density is 2634 kg/m3.
- The molar mass of SiO2 is 60.0843 g/mol.
- The melting point is 1986 K, whereas the boiling point is 2503 K.
- Its crystal structure is Quartz, cristobalite or tridymite.
Applications of Silicon Dioxide (SiO2) Nano Powder
Silica (SiO2) nano powder is used to make flat glass, glass products, molten sand, cement, fiberglass, ceramic enamel, sandblasting for antioxidants, filter sand, flux, refractory and light concrete. Silicon dioxide (SiO2) Nanoparticles is widely used in many industrial products. Rare crystals in nature can be used to create important parts of the electronics, optical instruments and crafts industry. Silicon dioxide (SiO2)nano powder is an important raw material for the manufacture of optical fibers. Generally, pure quartz can be used to make quartz glass. The coefficient of expansion of quartz glass is very small. It is equivalent to 1/18 of ordinary glass. It can withstand temperature change and acid resistance is good. Therefore, quartz glass is often used to make chemical instruments resistant to high temperatures. Quartz sand is often used as a glass material and as a building material.
The main applications and uses of Silicon dioxide (SiO2)Nanoparticles are stated below:
1. SiO2 Nanoparticles as Adhesive and Sealer
Silica (SiO2)nano powder is the preferred material in the adhesive and sealant field. Adding it to the sealant can quickly form a net structure, inhibit colloidal liquid, increases speed, improves the effect of bonding, and because the particles are small, the sealing of the adhesive increases.
Moreover, due to the special development of hydrophobic silicon dioxide, structural adhesives belonging to advanced technologies have been produced. These structural adhesives allow the joining of different materials such as steel, aluminum, magnesium, and plastic.
Numerous processes can benefit from the technical advantages and improvements that can be made with the proper selection of dioxides that have been specially developed for the special adhesives and sealants industry. Silicon dioxide (SiO2)nano powder not only improves the mechanical and rheological properties but also acts as a counter to the solution of agents that improve the storage and processing stability of adhesives and sealants.
2. SiO2 Nanoparticles in Material Packing
Silicon dioxide (SiO2) Nanoparticles has a three-dimensional network structure and a large specific surface area. They show great activity and can form a mesh structure when the paint dries, it increases the strength and finish of the coating and improves the suspended pigment of the paint which do not fade for a long time.
3. SiO2 Nanoparticles in Textile Industry
Silica (SiO2)nano powder has played an important role in functional textiles, at present, it has been used to prevent ultraviolet and works as an anti-aging and anti-bacterial deodorant. For example, the appropriate proportion of Silicon dioxide (SiO2)Nanoparticles is an important additive in resistance to ultraviolet radiation fiber.
4. SiO2 Nanoparticles in Catalysis
Due to its large specific surface area, high porosity and good surface activity, Silicon dioxide (SiO2)nano powder has potential applications in the catalysis. Silicon dioxide (SiO2)nano powder works as a catalyst carrier. It shows the unique response performance for many sensitive reactions.
5. SiO2 Nanoparticles in Bactericide field
Silica (SiO2)nano powder is used as a carrier in the preparation of fungicides, in which the antibacterial ion can be adsorbed and achieve the objective of sterilization. The application is used for the manufacture of items such as the refrigerator case, the computer keyboard. etc.
6. SiO2 Nanoparticles as Lubricating oil additives
Silicon dioxide (SiO2)Nanoparticles as lubricating oil additives show the excellent anti-wear property. In the field of lubricating oil additives, the particles of Silicon dioxide (SiO2)nano powder has hydroxyl and unsaturated bond and can form a solid chemical adsorption film and protect the metallic surface to significantly improve the friction performance of the lubricating oil.
7. SiO2 Nanoparticles as Reinforcing Agent
Silicon dioxide (SiO2)Nanoparticles can replace carbon black as a reinforcing agent. It is anti-aging and changes color in rubber, it can produce colorful adhesive on the side of the tire.
8. SiO2 Nanoparticles’ Biomedical applications
Silica (SiO2)Nanoparticles may be used in cellular functions. Silicon dioxide (SiO2)nano powder has good biocompatibility and, therefore, are widely used as biological carriers. It has been found that Silicon dioxide (SiO2)nano powder interfere with the passage of Wnt signaling, which affects biological processes such as adipocyte differentiation, cancer cell migration and the development of zebrafish embryos. Silica (SiO2)Nanoparticles can cause Wnt signaling molecules to access the lysosome and degradation, and other molecules of Wnt signaling is not affected.
9. SiO2 Nanoparticles in Pharmaceutical Industry
Silica (SiO2)nano powder has an absolute necessity in the pharmaceutical industry. Silicon dioxide has become one of the most important and frequently used pharmaceutical excipients. The improvement of the flow properties of the materials necessary for the modern manufacture of tablets and capsules is nothing more than an example of the advantages obtained by the use of Silicon dioxide (SiO2)nano powder.
Some of the advantages and benefits of Silicon dioxide (SiO2)Nanoparticles for the pharmaceutical industry are solid forms of drugs, semi-solid forms of drugs, liquid forms of drugs, HD Pharma grade and Pharmacopoeia monographs.
10. SiO2 Nanoparticles in Paints and inks
Silicon dioxide (SiO2)nano powder is mainly used in paints and coatings to control rheological characteristics, as a thixotropic agent, as opposed to the agent solution and to aid in the prevention of oxidation and Corrosion, this product should be added in concentrations of 0.5% – 2%.
11. SiO2 Nanoparticles’ Ceramic use
It is a glass former. It is incorporated into the glaze as ground quartz. It is part of feldspars, clays, as a soluble source in the form of sodium silicate, it is found in all frits as well as in ashes especially rice or reeds. It has little influence on colors, except chrome and nickel reds. Increases opacity in glazes is rich in zinc and borax.
12. SiO2 Nanoparticles in Agriculture and Food Industry
In agriculture, Silica (SiO2) Nanoparticles is used for seed treatment, can make vegetables, cabbage, tomatoes, yellow, cotton, corn, wheat, and increases the yield. Silicon dioxide (SiO2)nano powder can be used in herbicides and pesticides.
In the food industry, food packaging bags that add Silicon dioxide (SiO2)nano powder keeps fruits and vegetables fresh. It is also used as anticoagulant, antifoam, thickener, auxiliary filter and clarifier in the food industry. The hygienic standard for the use of food additives stipulates that it can be used in egg powder, powdered sugar, powdered milk, cocoa powder, cocoa butter, vegetable powder, instant coffee, powder of soup, etc. The maximum amount of use is 15 g/kg.
Products that dissolve in water sometimes show a delay in getting wet or create lumps when removed. A further advantage of the adhesion of a food-grade hydrophilic Silica is that it improves wettability and even helps to prevent lumps from forming.
This is because silicon dust is enveloping, thus acting as a holder of space between the individual and the dust particles. In the water spaces, silicon dioxide provides good retention of colored powders and high transparency once it is raised in the water.
Thus, the addition of silicon dioxide helps prevent agglomeration, allowing powder producers to permanently offer a high-quality product worldwide, therefore, food products treated with silicon dioxide show stability and long storage.
Moreover, Silica is added in powdered foods and other supplements so that other ingredients bind together; For example, food manufacturers add silicon dioxide to salt and various species to keep substances dry, as an anti-caking agent, and to make processed foods tastier.
It also prevents the accumulation of some toxins in the body, such as aluminum and other heavy metals, which can prevent some problems that arise, as well as diseases when there is an acidic environment in many organs; the brain is one of them, which needs a good natural dosage to work properly over the years. Obviously, silicon dioxide is important to keep the musculoskeletal system healthy, especially during periods of training, where an extra agent is required to mineralize muscle collagen and bone system.
When the nails are brittle, a silicon dioxide supplement may be enough to recover them and keep them healthy. Taking silicon dioxide as a support agent for joint maintenance can be relatively useful. An investigation showed that by supplementing the diet with SiO2 as compared to other protective agents of the joints, this compound shows its power by keeping the entire system stronger in the face of hard training, in addition to helping to have a much more pronounced mental approach.
In summary, Silicon dioxide (SiO2) Nanoparticles is a compound of silicon and oxygen, commonly called silica. It is one of the components of the sand. In nature, it occurs naturally as quartz. This spatially composed compound in a three-dimensional (crystallized) network forms quartz and all its varieties. If it is in an amorphous state it constitutes the opal and usually includes a high percentage of water. Silica (SiO2) nano powder has countless applications in a variety of sectors. It is used for the manufacturing of glass, quartz glass, soluble glass, optical fiber, important components of the electronics industry, optical instruments, crafts, and refractory materials. Moreover, it can act as reinforcing agent, adhesive, sealer and much more. It can be found naturally in Quartz. In fact, you will find its applications everywhere!
Iron Oxide Nanoparticles and Applications
Iron oxide nanoparticles/nanopowder are nanoscale crystals of iron oxide (g-Fe2O3, α-Fe2O3 and Fe3O4) with a considerably great surface area and surface-to-volume ratio. Iron oxide has a unique superparamagnetic property with an easy separation method. Considering their physical and chemical specifications, iron oxide nanoparticles have biomedical, environmental and agricultural applications. Iron oxide nanoparticles/nanopowder are advantageous as they can be modified by organic and inorganic agents, antibodies, nonionic detergents, starches, enzymes, proteins, drugs, nucleotides and polyelectrolytes for further applications based on their wide surface chemistry potentials.
Synthesis of Iron Nanoparticles
Several methods have been suggested for the synthesis of iron nanoparticles namely dry processes, microbiological techniques and wet chemistry. Generally, the synthesis methods can be categorized as physical, chemical and biological methods. In the physical method of iron oxide nanoparticle synthesis, the top-down technique is adopted to breakdown the bulky crystals into smaller ones. Nevertheless, the control over the particles size and homogeneity is lost and some particles get sizes that don’t fit in the nanoscales. Some of the physical techniques are laser ablation, ultraviolet irradiation, lithography, ultrasonic fields and aerosol technologies. The chemical synthesis methods are considered the best and most efficient with higher uniformity of particles in nanoscales. These methods are simple and tractable through which the particles shape and composition can be controlled so precisely. Amongst, liquid phase methods, two-phase methods (microemulsion), sol–gel method, gas/aerosol phase method, polyols method, hydrothermal reaction methods and sonolysis are the methods with favorable products. Briefly, the iron oxide nanoparticles are prepared through coprecipitation reaction of Fe2+ and Fe3+ through adding a base. The type of the iron slat used in the synthesis process, ionic strength, pH and the Fe2+/Fe3+ ratio determine the composition, shape and size of the resulting nanoparticles. The biological method of iron oxide nanoparticles synthesis is also advantageous as it fits well in the green chemistry promises through using microbes, bacteria, viruses and fungi in the synthesis process. Such biological methods include intracellular synthesis of nanoparticles by bacteria, extracellular synthesis of nanoparticles by bacteria, biosynthesis of nanoparticles by fungi, intracellular synthesis of nanoparticles by fungi, actinomycete mediated synthesis of nanoparticles, yeast mediated synthesis of nanoparticles and virus mediated biosynthesis of nanoparticles 31.
Surface Modification of Iron Oxide Nanoparticles
With all the advantageous of iron oxide nanoparticles, they suffer from agglomeration when they are left unmodified mainly because of their high-energy surface, van der Waals forces and strong magnetic power. In addition to this, higher concentration of Fe2+ and Fe3+ is considered toxic for organism. However, if the surface of the nanoparticles is modified or functionalized, it is easy to get over these consequences. Modification and functionalization increase the compatibility of the iron oxide nanoparticles and creates hydrophilicity on their surface. Therefore, a careful and smart surface modification strategy can increase the selectivity in delivering a given drug or agent to a particular organ and more importantly, make the iron oxide nanoparticles biocompatible and nontoxic. Following the necessities mentioned above, there have been a lot of efforts towards coating and modifying the iron oxide nanoparticles/ nanoparticles/nanopowder. In biomedical application, the modification is according on the application. It has been found out that proteins, enzymes, antibodies, nucleotides and drugs can bind to magnetic iron oxide nanoparticles. There are reports of coating iron oxide nanoparticles with biomolecules such as lactobiotonic acid, gluconic acid and polyacrylic acid with the resulting homogenous particle size distribution and hydrophilic properties for uses in tissue engineering and liposome coating 1.
Important Factors to Enhance the Efficiency of Iron Oxide Nanoparticles
First of all, chemical precipitation is regarded as the cheapest, simplest and the most effective way to synthesize magnetic iron oxide nanoparticles. Next, the use of economical initial reagents and chemicals can lead to cost-effective final iron oxide nanoparticles for applications in less sensitive purposes. Later, the small size of iron oxide nanoparticles results in high surface area to volume ratio enabling interactions with distinct chemical specious as aqueous and gaseous ones. Finally, it is possible to activate iron oxide nanoparticles through altering the particle shape to make the catalytic site more available. Therefore, reproducible, scalable, durable and dispersible particles with controlled growth, shape and nucleation are significant 1.
Boron Nitride Nanoparticles
Boron nitride, consists of Boron and Nitrogen, is a heat and chemically resistant chemical compound with the chemical formula BN. Because of excellent thermal and chemical stability, boron nitride ceramics are traditionally used as parts of high-temperature equipment.
Boron Nitride has four different structures that differ in the arrangement of boron and nitrogen atoms, but the most stable crystalline form of this compound is hexagonal form.
Zinc Sputtering Targets and Applications
Zinc is a chemical element with symbol Zn and atomic number 30. Zinc is the 24th most abundant element in Earth’s crust and has five stable isotopes.
Zinc is a metal that is used in many applications in many different ways. By the advance in technology zinc sputtering targets are started to be used to make thin films and have outstanding properties. The most important area that zinc sputtering targets can be used is galvanization. As all we may know by the moisture in the air we face with the problem of corrosion. For the solution of this problem zinc help us. We can product automobiles, bridges and ships by coating them with zinc sputtering targets.
We can also make zinc alloys with other metals to have more intensity and hardness. By this way zinc becomes a suitable material to be used in automobile manufacturing and the mechanical industry, thanks to its superior superplasticity.
Since zinc has good electromagnetic field resistance properties, thin zinc films obtained by sputtering targets are very effective shielding material.
And as zinc is non-magnetic, it is suitable for making components and covers of instruments and meters.
To make explosion-proof equipments zinc sputtering targets can help you due to the properties of zinc which produces no sparks, either alone or in collision with other metals.
Graphene Oxide: Descriptions, Usage and Applications
Carbon atoms bounded together to form repeated hexagon patterns is called Graphene, and it is considered as 2-d crystal structure. Due to its shape, it has unique properties such as both the strongest, and the lightest material in the world, most conductive, and transparent.
Although the graphene is expensive and relatively hard to manufacture, effective and inexpensive graphene derivatives can be produced. Graphene oxide (GO) is one of these, and it is oxidized form of graphene, so one can make graphene via dispersion of graphene oxide in water and also other solvents. Graphene oxide is not a good conductor even it is considered as electrical insulator, but functionalization can be done depending on the application such as optoelectronics, bio-devices, and drug-delivery systems.
Single-walled carbon nanotube semiconductors could be favorable for photovoltaic systems.
Researchers at the Energy Department’s National Renewable Energy Laboratory (NREL) discovered single-walled carbon nanotube semiconductors could be favorable for photovoltaic systems because they can potentially convert sunlight to electricity or fuels without losing much energy.
The research builds on the Nobel Prize-winning work of Rudolph Marcus, who developed a fundamental tenet of physical chemistry that explains the rate at which an electron can move from one chemical to another. The Marcus formulation, however, has rarely been used to study photoinduced electron transfer for emerging organic semiconductors such as single-walled carbon nanotubes (SWCNT) that can be used in organic PV devices.
To read more about the work being done by Jeffrey Blackburn, a senior scientist at NREL and the team from Colorado State University go here. To visit Skyspring Nanomaterials, Inc. and learn more about our single-walled, carbon nanotubes go here.
Aluminum Paste
Aluminium Test Laboratory
Tests and measurements are made necessary for preparing of an optimum recipe for Aluminium Paste in our laboratory. Each batch is constantly analyzed to ensure a high quality product delivery. Active aluminum, Hydrogen Gas Output Curve and laser fineness / particle distribution are analyzed as standard Aluminum Paste analysis.
Safety
The most outstanding feature of separating Aluminum Paste from other products is not an explosion risk because of in powder form. Aluminum Paste is not flammable. Aluminum Paste water based and does not contain organic solvents. ALUPORE does not effect a threat to human health during transportation, storage and use. Aluminum Paste is not scope of ADR regulations related with the transportation of dangerous materials.
Dispatch
Economical transport solutions are offered and Aluminium Paste is prepared to respond to the needs of our customers in appropriate packaging.
Alpha Gypsum
Alpha Gypsum is obtained by heating of gypsum stone under high pressure and Alpha Gypsum is chemically same with CaSO4.1 / 2H2O formula and beta gypsum but different from in the point of crystal structure. Structure of beta gypsum is needle-like and amorphous while crystal structure of Alpha Gypsum is geometric. It shows that Alpha Gypsum as compare to beta gypsum appears to be fluidized when mixed with much less water than beta gypsum and Alpha Gypsum offers high strength properties.
Alpha Gypsum is preferred in different industrial applications by virtue of high mechanical strength.
Application Areas:
- Ceramic Molding Plaster
- Dental Plaster
- Screed Binder
- Construction Chemicals
- Orthopedic Plaster
- Architectural and Decorative Adornments