Gold Nanoparticles Against Pathogens of Coronavirus Family
Coronavirus (2019-nCoV) researches continue to find a remedy for the spread. All disciplines are working in a coordination to stop this disease. There are test, vaccine, and treatment researches all around world while time is ticking. However, answer might be in nanotechnology to find COVID-19 test, or to develop a vaccine for coronavirus. Below, we dived into the possible scenario of using gold nanoparticle to fight against coronavirus family and other nanotechnological contributions to halt novel Coronavirus.
Coronavirus pathogens including SARS-CoV that stands for sever acute respiratory syndrome coronavirus, SARS-CoV-2 which is responsible for coronavirus disease 2019 (COVID-19) and Middle East respiratory syndrome (MERS-CoV) cause life-threatening pneumonia in humans with almost no licensed and specific therapeutic vaccines so far. In the case of COVID-19, which is the most contagious and deadliest coronavirus disease with nearly two million infected people worldwide and over 140 thousand deaths, the 70-yearl-old malaria drug, chloroquine has shown to have some confirmed therapeutic effects, lately. However, some clinical reports have confirmed that a quarter of patients with COVID-19 have developed heart disease. Moreover, several vaccines which take the advantage of nanoparticles have been introduced to fight MERS-CoV. Even though, there are few antiviral drugs and vaccines for therapeutic purposes, poor metabolic stability, serious adverse health effects and low antiviral activities have greatly impaired their remedial effects 1. Consequently, there is an urgent need to develop practical and effective treatment for the newly arisen COVID-19.
Click Image to Reach Our Other Articles About COVID-19
Coronavirus Pathogens Binding and Fusion Mechanism
Prior to any mechanistic discussion over the pathogens of coronavirus family, it is helpful to review the molecular structure and types of protein they consist of. Coronaviruses are a family of enveloped positive-stranded RNA viruses comprising 26 to 32 kilobase genome which is regarded as the largest among the RNA of all viruses. They are found among an extensive range of hosts capable of infecting species like mammalians and birds as well as serious health disorders such as gastrointestinal, respiratory, hepatic and central nervous system diseases. In coronaviruses, the spike protein forms crown-like (Corona in Latin) structures coming out of the virus surface used to penetrate the host cells. The spike protein itself comprises S1 and S2 where the S1 binds to host cell receptor and S2 for membrane fusion. The nucleocapsid protein is considered as the most abundant protein in coronavirus and a highly immunogenic phosphoprotein. It should be noted that the nucleocapsid protein hardly ever does mutate and is used as a marker in diagnostic assays 2.
The mechanism through which coronavirus pathogens bind to cellular receptors with a consequent membrane fusion mediation is the use of the spike (S) protein. An increasing amount of studies and experiments demonstrate the S protein involvement in the infection. The S1 proteins as it was introduced above, binds to host cells through dipeptidyl peptidase 4 receptors (DPP4) with the consecutive triggering of S2 protein in order to mediate the fusion of the virus envelope with host cell membrane. The S2 protein’s censorious role to regulate the infection by coronaviruses demonstrates its inhibition function is a powerful technique in treatment. Specifically, the S2 protein is made of three main areas including heptad repeat 1 (HR1), heptad repeat 2 (HR2) and fusion peptides (FP). After the S1 proteins binding to the DPP4 is done, the fusion peptide goes through the membrane of the host cells. Later, the HR1 and HR2 twist around each other forming a helical structure called 6-helix bundle (6-HB) which itself is responsible for drawing the host cell membrane and the envelope of the virus toward each other and promotes their fusion. This fusion results in the release of the viral genome (RNA) into the host cell. According to this mechanism, 6-HB formation seems to be a critical factor in mediating the coronavirus fusion with cell membranes 1.
Click Image to Find Out More About Nanomaterials in Medicine
Currently, the most possible strategy against the spike protein is employing antibodies that are capable of blocking the virus binding and fusion in order to neutralize the infection. As a result, the spike (S) protein has to be regarded as a drug or vaccine target candidate to suppress the infectious process and replications. Studies and experiments conducted on animals suggest that any inadequate protective immune system against the pathogens of coronavirus can lead to an eosinophilic immunopathology in the lungs when the body is infected 3.
Since the SARS-CoV-2 is a newly emerged pathogen from the coronavirus family and there are very few treatments based on nanotechnology, the upcoming section will specifically focus on the previously developed gold nanoparticle-based treatment methods against SARS-CoV and MERS-CoV. However, a precise review of the past studies can help researchers come up with novel methods to specifically deal with the pandemic COVID-19 today that has claimed thousands of lives across the world.
Gold Nanoparticles for Detection and Inhibition of Coronavirus Pathogens
Colorimetric techniques have shown to serve as effective tools to detect and identify target analytes through the change in the color of an indicator such as a metallic nanoparticle and fluorescent molecules. The detection involves approving a biomarkers attendance or presence based on the visual observation through measuring the absorbance of the dye agent at a particular characteristic wavelength. Based on a recent study carried out in early 2019, a colorimetric assay as a detection platform was developed to detect MERS-CoV using double-strand DNA (dsDNA) self-assembly coated or shielded with gold nanoparticles in magnesium chloride as a positive electrolyte. This platform is capable of detecting MERS-CoV by the change in the color gold nanoparticles in the ultra violet and visible (UV-Visible) wavelength range and the shift in their local surface Plasmon resonance (LSPR). Accordingly, a pair of probes at the 5′ end or 3′ end of the double-stranded DNA is designed and modified with thiol in order to organize complementary base pair with upstream of the E protein gene and open reading frames (ORF) on MERS-CoV. In this order, the probes and the targets’ dsDNA form a disulfide-induced long self-assembled complex to protect the gold nanoparticles from transition of optical properties and self-induced aggregation. This colorimetric detection platform can discriminate 1 to Pico molar per one micro liter (1 pM/µL) of MERS-CoV 4. Regarding the similarities in structure, this platform can potentially be employed to develop SARS-CoV-2 kits with some modification.
Gold Nanoparticle-Modified Spike Protein as a Potent Antigen against Coronavirus
Gold nanoparticles, which are believed to act as an adjuvant and an antigen carrier in immunization simultaneously, are considered as an effective adjuvant in SARS-CoV vaccine which is inactivated by ultraviolet radiation. In this method, the preliminary tests were conducted on mice through immunizing them by 0.5 µg spike protein (S) in the absence of adjuvant SARS after the infection is done with mouse-adapted virus. The protein that is adjuvanted with gold nanoparticles lead to a potent IgG response but fails to increase the efficacy of the vaccine or stop/decrease the eosinophilic infiltration. Based on the results of this study, gold nanoparticle‐adjuvanted S protein can result in a promoted antigen-specific IgG response against SARS-CoV 3.
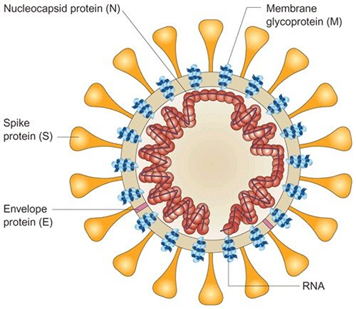
Figure 1. Schematic Diagram of Coronavirus
HR1 Peptide Inhibitor Based on Gold Nanorod
Taking the advantage of gold nanorods in this method, a set of inhibitors of heptad repeat 1 (HR1) has been developed in order to prevent HR1/HR2-mediated membrane fusion between the host cells and MERS-CoV. Specifically in this technique, peptide pregnancy-induced hypertension (PIH), which has turned out to show strong inhibition against MERS-CoV, becomes even 10 times stronger when it forms a complex with gold nanorods (PIH-AuNRs) exhibiting an additional promoted biocompatibility and metabolic stability. This complexation has shown a novel antiviral activity with a potential quality to be used in developing clinical forms as drugs or vaccine in treating MERS-CoV infection 1.
Conclusion
With regard to the structural similarities among the pathogens of coronavirus including MERS-CoV, SARS-CoV and the novel SARS-CoV-2, gold nanoparticles can not only serve as treatment strategy against COVID-19, but they can be applied to design coronavirus diagnostic kits. As the previous studies have proved the satisfactory results gold nanoparticles applications, studies concerning developing AuNP-based platforms can emerge as a relief in the critical moments the world has gone through.
References
1. Huang, X. et al. Novel Gold Nanorod-Based HR1 Peptide Inhibitor for Middle East Respiratory Syndrome Coronavirus. ACS Appl. Mater. Interfaces 11, 19799–19807 (2019).
2. Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 3, 237–261 (2016).
3. Sekimukai, H. et al. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 64, 33–51 (2020).
4. Kim, H. et al. Development of Label-Free Colorimetric Assay for MERS-CoV Using Gold Nanoparticles. ACS Sensors 4, 1306–1312 (2019).
You must be logged in to post a comment.




About the author