Copper Nanoparticles
Over the past few years, a considerable interest has been focused on metal nanoparticles due to their potential applications in diverse fields including catalysis, magnetic recording media, or microelectronics. The metal nanoparticles are finding applications in optoelectronics, nanodevices, nanoelectronics, nano-sensors, information storage and catalysis. Among various metal particles, copper nanoparticles have attracted more attention because of their catalytic, optical, electrical and antifungal/antibacterial applications. Copper nanoparticles are being researched a lot due to its high electrical conductivity, high melting point, low electrochemical migration behavior, excellent solderability and low material cost. Copper is one of the most widely used materials in the world. It has a great significance in all industries, particularly in the electrical industry due to its low cost.
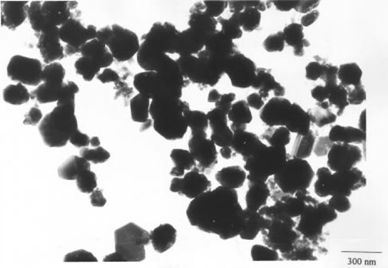
Figure 1: TEM image of copper nanoparticles[1].
Production of Copper Nanoparticles
The synthesis of nanoparticles has received considerable attention because of their unique physical and chemical properties that are distinct from the bulk materials. Various methods are available for synthesis of copper nanoparticles. Copper nanoparticles received much attention because of large volume of literatures concerning the production and characterization of copper nanoparticles. Several methods have been proposed for synthesis of copper nanoparticles by considering bottom-up and top-down methods. The major techniques for preparing copper nanoparticles by chemical methods are chemical reduction, sonochemical reduction, micro emulsion techniques, electrochemical, hydrothermal, sol gel synthesis, polyol process, biological synthesis and microwave assisted techniques. Physical methods for the synthesis of copper nanoparticles are laser ablation, vapor phase synthesis, mechanical milling and pulsed wire discharge. Chemical methods of preparing copper nanoparticles are simple in operation, high exile and environment friendly. In electrochemical method, the copper nanoparticles can be produced at room temperature without using any additives. The main advantages of powder production by electrolysis are high purity and there is no need to use high pressure, toxic chemicals and vacuum systems to produce the product.
Click Image to Learn More About Top-Down Bottom-Up Approach
Copper nanoparticles can be produced by using various techniques typically classified as bottom-up or chemical methods and top-down or physical methodswhich is shown in Fig. 1. The structure of copper nanoparticles is composed of atoms and clusters or molecules in bottom-up approach where as in the case of top-down approach a bulk piece of required material diminished to nanoscale dimensions using mechanical milling, cutting, etching and grinding techniques. Some of the methods like chemical reduction,sonochemical reduction, microemulsion techniques,electrochemical,hydrothermal, sol–gel synthesis,polyol process and microwave-assisted methodsare the major techniques for preparing copper nanoparticles from bottom-up approach. Biological syntheses are also treated as bottom-up approach.Top down methods for synthesis of copper nanoparticles are laser ablation,vapor phase synthesis,mechanical millingand pulsed wire discharge (PWD).
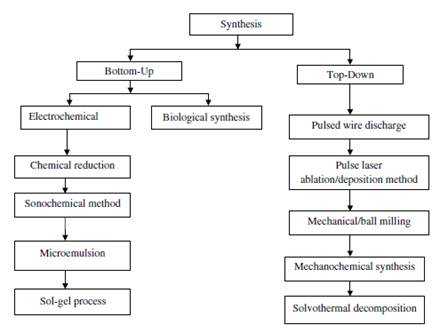
Figure 2: Classification of synthesis techniques of copper nanoparticles[2].
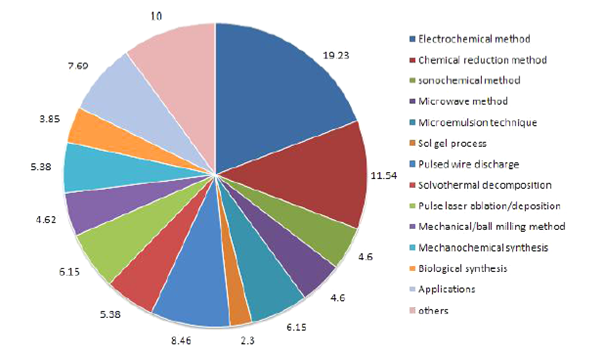
Figure 3: Pie chart for synthesis of copper nanoparticles[3].
Applications
Copper nanoparticles have received considerable attention due to their physicochemical properties such as high melting point temperature, magnetism, electrical and thermal conductivity, light absorption and high heat transfer. Copper nanoparticles are used as an antimicrobial agent due to their high surface-to-volume ratio and easy interaction with other particles to enhance their antimicrobial efficiency. Copper nanoparticles are highly reactive compared to other metallic nanoparticles. Due to these special properties and small dimensions, copper nanoparticles hold important application in heat transfer systems, sensors, high strength materials, catalysts, antimicrobial materials, etc.
The usage of biodiesel in automotive engines is limited due to its low performance and high nitrogen oxide emission. Nano-copper particles was used as the fuel additive for diesel engine with Soya bean biodiesel (B10). The copper nanoparticles were prepared using electrochemical method and the size of Cu nanoparticles were in the range of 40–50 nm. Nano-copper particles exhibits better engine performance and reduced nitrogen oxide emission when compared to other formulations.
Anti-microbial effects of metal nanoparticles received considerable attention from both medical and technological point of view. Metallic Cu particles are used as an anti-infective agent for replacing Ag and other noble metal composites and finds applications such as water treatment, food processing and protection of medical instruments. Glucosamine-functionalized copper nanoparticles synthesized from injection pump-assisted reaction environment and in situ reflux condensation exhibits outstanding anti-bacterial activity against two Gram-negative and Gram-positive strain. The dynamic and structural light scattering reveals that glucosamine functionalized copper nanoparticles are spherical in shape with hybrid morphology.
Metallic copper nanoparticles and its composites provide flexible interface activity in biological and biosensing applications due its special properties like bio compatibility, high stability against aggregation and hydrophilic character. Glucosaminesurface-modified copper nanoparticles prepared by integration of injection pump and ultra-sonochemistry.
Click Image to Learn About Copper
Even though bulk microstructures and the nanostructure of metallic copper demonstrate distinct applications, the functionalized copper nanoparticles exhibit advanced features such as high thermal stability, electrochemical feasibility toward biomolecule (C-reactive protein, CRP) detection and more crystallinity. The development of miniaturized nanodevices that incorporates electronic, chemical, photonic and biological features is essential for future electronic and sensing devices. The photosensitivity and extensive cross section of metal nanoparticles makes them highly applicable in several applications such as optical sensors and in surface enhanced spectroscopes. Cu nanoparticles prepared by nanosphere lithography may potentially be used for replacing expensive metals of Ag and Au in certain applications.
Electrochemical capacitors are received considerable attention because of their long-life cycle, high power density and excellent reversibility. Copper oxide multilayer nanosheets prepared by chemical bath deposition method shows hydrophilic nature with water and the copper oxide electrode exhibits super capacitance. This shows copper oxide thin films are most suitable electrode material for electrochemical capacitors. Anti-microbial effects of metal nanoparticles received considerable attention from both medical and technological point of view. Metallic Cu particles was used as an anti-infective agent for replacing Ag and other noble metal composites and finds applications such as water treatment, food processing and protection of medical instruments. Glucosamine-functionalized copper nanoparticles synthesized from injection pump-assisted reaction environment and in situ reflux condensation exhibits outstanding anti-bacterial activity against two Gram-negative and Gram-positive strain. The dynamic and structural light scattering reveals that glucosamine functionalized copper nanoparticles are spherical in shape with hybrid morphology.
Metallic copper nanoparticles and its composites provide flexible interface activity in biological and biosensing applications due its special properties like bio compatibility, high stability against aggregation and hydrophilic character. Glucosaminesurface-modified copper nanoparticles prepared by integration of injection pump and ultrasonochemistry. Even though bulk microstructures and the nanostructure of metallic copper demonstrate distinct applications, the functionalized copper nanoparticles exhibit advanced features such as high thermal stability, electrochemical feasibility toward biomolecule (C-reactive protein, CRP) detection and more crystallinity.
The development of miniaturized nanodevices that incorporates electronic, chemical, photonic and biological features is essential for future electronic and sensing devices. The photosensitivity and extensive cross section of metal nanoparticles makes them highly applicable in several applications such as optical sensors and in surface enhanced spectroscopes.
Cu nanoparticles prepared by nanosphere lithography may potentially be used for replacing expensive metals of Ag and Au in certain applications. Electrochemical capacitors are received considerable attention because of their long-life cycle, high power density and excellent reversibility. Copper oxide multilayer nanosheets prepared by chemical bath deposition method shows hydrophilic nature with water and the copper oxide electrode exhibits super capacitance. This shows copper oxide thin films are most suitable electrode material for electrochemical capacitors.
Physical method of preparing nanoparticles requires expensive equipment, high temperature and vacuum systems. Biological methods are also used for synthesis of copper nanoparticles, but due to lack of knowledge and experience it is not applicable to produce nanoparticles. Chemical methods are employed for synthesis of nanoparticles due to its low cost, simple operation, high edibility, easy availability of equipment, no need of vacuum systems, environment friendly and provides high yield in ambient conditions.
In a general way, it can be concluded that the formation of Cu-NPs is extremely sensitive to the synthesis conditions. Therefore, before using a synthesis method described in the literature, one should observe the real influence of synthesis conditions such as temperature, stirring and composition of the mixture for an effective control of size distribution and particle shape. Also, it should be considered that the metallic nanoparticles are rather a new research subject in science world. Although cobalt nanoparticles have some outstanding properties, it is a rather new material and more research should be done in order to enhance the properties and moderate the disadvantages. A lot of researches are still being conducted to improve its properties and lower its manufacturing cost in order to implement its use in real life applications. In the coming years, this material will innovate the world of technology due to its unique characteristics.
REFERENCES
1.P.K. Khanna, S. Gaikwad, P.V. Adhyapak, N. Singh, R. Marimuthu, Synthesis and characterization of copper nanoparticles, Materials Letters, 13 March 2007.
2.Jeyaraman Ramyadevi, Kadarkaraithangam Jeyasubramanian, Arumugam Marikani,
Govindasamy Rajakumar, Abdul Abdul Rahuman, Synthesis and antimicrobial activity of copper nanoparticles, Materials Letters, 19 December 2011.
3.N. Arul Dhas, C. Paul Raj, and A. Gedanken, Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles, Department of Chemistry, Bar-Ilan University, 25 February 1988.
4.A. Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
5.R. Bali, N. Razak, A. Lumb and A. T. Harris, The synthesis of metallic nanoparticles inside live plants. Int. Conference on Nanoscience and Nanotechnology, 2006. ICONN ’06., Brisbane, Qld, (2006).
6.Yanhu Wei, Steven Chen, Bartlomiej Kowalczyk, Sabil Huda, Timothy P. Gray, and Bartosz A. Grzybowski, Synthesis of Stable, Low-Dispersity Copper Nanoparticles and Nanorods and Their Antifungal and Catalytic Properties,Department of Chemical and Biological Engineering, Department of Chemistry, Northwestern UniVersity, 31 July 2010.
REFERENCES FOR IMAGES
1.N. Arul Dhas, C. Paul Raj, and A. Gedanken, Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles, Department of Chemistry, Bar-Ilan University, 25 February 1988.
2.Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
3.Tamilvanan, K. Balamurugan, K. Ponappa, Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application, International Journal of Nanoscience, 20 May 2014.
What is copper powder?
What is copper powder?
Characteristics: Distinctive reddish color; ductile; excellent conductor of electricity. Complexing agent, coordination numbers 2 and 4. Dissolves readily in nitric and hot concentrated H2SO4, in HCl and dilute H2SO4 slowly but only when exposed to the atmosphere. More resistant to atmospheric corrosion than iron, forming a green layer of hydrated basic carbonate. Readily attacked by alkalies. A necessary trace element in human diet; a factor in plant metabolism. Essentially nontoxic in elemental form. Powder is combustible.
Derivation: With sulfide ores the steps may be
(1) concentration (of low grade ores) by flotation and leaching
(2) roasting
(3) formation of copper “matte” (40-50% Cu)
(4) reduction of matte to “blister” copper (96-98%)
(5) electrolytic refining to 99.9+% copper
Hazard: Toxic and flammable in finely divided form.
Tolerance (fume) 0.2 mg/m3; (dusts and mists) 1 mg/m3.
Using Copper Powders
Copper and copper alloy powders have been used in industrial applications for many years. Probably the best known is the self-lubricating bearing which was the first major application and still accounts for about 70% of the granular copper powder used. This application takes advantage of the ability to produce a component with controlled interconnected and surface-connected porosity. The production of metallic filters also takes advantage of this ability.
Pure copper powder is used in the electrical and the electronics industries because of its excellent electrical and thermal conductivities. Alloyed with tin, zinc, nickel and other elements, copper in powder form is used in structural parts and friction materials. Brasses, bronzes and other copper alloys produced by powder metallurgy methods have the physical and mechanical properties of their cast or wrought counterparts. Copper is used also as an alloying element in iron powder components to enhance the mechanical properties and control dimensional changes during sintering, the addition being made either by mixing or by infiltration.
In addition to the above applications of granular copper powder, a large quantity of copper and copper alloy powder is used in flake form,i.e., as a powder whose thickness is small in relation to its other dimensions. Such powders are used, for example, in antifouling paints, decorative and protective coatings and printing inks.
Copper and copper alloy powders are also used in such nonstructural applications as brazing, cold soldering, and mechanical plating, as well as for medals and medallions, metal-plastic decorative products and a variety of chemical and medical purposes.